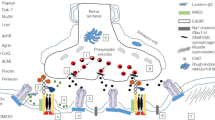
Abstract
The past decade saw remarkable advances in defining the molecular and genetic basis of the congenital myasthenic syndromes. These advances would not have been possible without antecedent clinical observations, electrophysiologic analysis, and careful morphologic studies that pointed to candidate genes or proteins. For example, a kinetic abnormality of the acetylcholine receptor (AChR) detected at the single channel level pointed to a kinetic mutation in an AChR subunit; endplate AChR deficiency suggested mutations residing in an AChR subunit or in rapsyn; absence of acetylcholinesterase (AChE) from the endplate predicted mutations in the catalytic or collagen-tailed subunit of this enzyme; and a history of abrupt episodes of apnea associated with a stimulation dependent decrease of endplate potentials and currents implicated proteins concerned with ACh resynthesis or vesicular filling. Discovery of mutations in endplate-specific proteins also prompted expression studies that afforded proof of pathogenicity, provided clues for rational therapy, lead to precise structure function correlations, and highlighted functionally significant residues or molecular domains that previous systematic mutagenesis studies had failed to detect. An overview of the spectrum of the congenital myasthenic syndromes suggests that most are caused by mutations in AChR subunits, and particularly in the ɛ subunit. Future studies will likely uncover new types of CMS that reside in molecules governing quantal release, organization of the synaptic basal lamina, and expression and aggregation of AChR on the postsynaptic junctional folds.
Similar content being viewed by others
References
Engel A. G, Ohno K., and Sine S. M. (1999) Congenital myasthenic syndromes, in Myasthenia Gravis and Myasthenic Disorders (Engel A. G., eds.) Oxford University Press, New York, pp. 251–297.
Ohno K., Tsujino A., Brengman J. M., Harper C. M., Bajzer Z., Udd B., Beyring R., Robb S., Kirkham F. J., and Engel A. G. (2001) Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc. Natl. Acad. Sci. USA 98, 2017–2022.
Walls T. J., Engel A. G., Nagel A. S., Harper C. M., and Trastek V. F. (1993) Congenital myasthenic syndrome associated with paucity of synaptic vesicles and reduced quantal release. Ann NY Acad. Sci. 681, 461–468.
Bady B., Chauplannaz G., and Carrier H. (1987) Congenital Lambert-Eaton myasthenic syndrome. J. Neurol. Neurosurg. Psychiatry 50, 476–478.
Maselli R. A., Kong D. Z., Bowe C. M., McDonald C. M., Ellis W. G., Agius M. A., Gomez C. M., Richman D. P., and Wollman R. L. (2001) Presynaptic congenital myasthenic syndrome due to quantal release deficiency. Neurology 57, 279–289.
Mora M., Lambert E. H., and Engel A. G. (1987) Synaptic vesicle abnormality in familial infantile myasthenia. Neurology 37, 206–214.
Byring R. F., Pihko H., Shen X-M, et al. (2002) Congenital myasthenic syndrome associated with epsiodic apnea and sudden infant death. Neuromuscul. Disord. 12, 548–553.
Okuda T., Haga T., Kanai Y., Endou H., Ishihara T., and Katsura I. (2000) Identification and characterization of the high-affinity choline transporter. Nature Neurosci. 3, 120–125.
Apparasundaram S., Ferguson S. M., George A. L. Jr., and Blakely R. D. (2000) Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem. Biophys. Res. Commun. 276, 862–867.
Oda Y., Nakanishi I., and Deguchi T. (1992) A complementary DNA for human choline acetyltransferase induces two forms of enzyme with different molecular weights in cultured cells. Brain Res. Mol. Brain Res. 16, 287–294.
Erickson J. D., Varoqui H., Eiden L. E., Schafer M. K., Modi W., Diebler M., Weihe E., Rand J., Bonner T. I., and Usdin T. B. (1994) Functional identification of a vesicular acetylcholine transporter and its expression from a ‘cholinergic’ gene locus. J. Biol. Chem. 269, 21,929–21,932.
Reimer R. J., Fon A. E., and Edwards R. H. (1998) Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr. Opin. Neurobiol. 8, 405–412.
Eiden L. E. (1998) The cholinergic gene locus. J. Neurochem. 70, 2227–2240.
Bööj S., Larsson P.-A., Dahllöf A.-G., and Dahlström A. (1986) Axonal transport of synapsin I and cholinergic synaptic vesicle-like material. Further immunohistochemical evidence for transport of axonal cholinergic transmitter vesicles in motor neurons. Acta. Physiol. Scand. 128, 155–165.
Kiene L.-M., and Stadler H. (1987) Synaptic vesicles in electromotoneurones. I. Axonal transport, site of transmitter uptake and processing of a core proteoglycan during maturation. EMBO J. 6, 2209–2215.
Llinás R., Sugimori M., Lin J.-W., Leopold P.-L., and Brady S. T. (1989) ATP-dependent directional movement of rat synaptic vesicles injected into the presynaptic terminal of squid giant synapse. Proc. Natl. Acad. Sci. USA. 86, 5656–5660.
Okada Y., Yamazaki H., Sekine-Aizawa Y., and Hirokawa N. (1995) The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell 81, 769–780.
Südhof T. C., and Jahn R. (1991) Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron 6, 665–677.
Engel A. G., Lambert E. H., and Gomez M. R. (1997) A new myasthenic syndrome with endplate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann. Neurol. 1, 315–330.
Bon S., Coussen F., and Massoulié J. (1997) Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J. Biol. Chem. 272, 3016–3021.
Deprez P. N., and Inestrosa N. C. (1995) Two heparin-binding domains are present on the collagenic tail of asymmetric acetylcholinesterase. J. Biol. Chem. 270, 11,043–11,046.
Ohno K., Engel A., G., Brengman J. M., Harper C. M., Shen X.-M., Heidenreich F. R., Vincent A., Milone M., Tan E., Demirci M., Walsh P., Nakano S., and Akiguchi I. (2000) The spectrum of mutations causing endplate acetylcholinesterase deficiency. Ann. Neurol. 47, 162–170.
Kimbell L. M., Ohno K., Rotundo R. L., and Engel A. G. (2001) Transplanting mutant human collagenic tailed acetylcholinesterase onto the frog neuromuscular junction: Evidence for an attachment defect in a congenital myasthenic syndrome. (Abstract) Mol. Biol. Cell 12(Suppl), 161a.
Prockop D. J., and Kivirikko K. I. (1995) Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434.
Ohno K., Brengman J. M., Tsujino A., and Engel A. G. (1998) Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc. Natl. Acad. Sci. USA 95, 9654–9659.
Donger C., Krejci E., Serradell P., Eymard B., Bon S., Nicole S., Chateau D., Gary F., Fardeau M., Massoulié J., and Guicheney P. (1998) Mutation in the human acetylcholinesterase-associated gene, COLQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency. Am. J. Hum. Genet. 63, 967–975.
Ohno K., Brengman J. M., Felice K. J., Cornblath D. R., and Engel A. G. (1999) Congenital endplate acetylcholinesterase deficiency caused by a nonsense mutation and an A-to-G splice site mutation at position +3 of the collagen-like tail subunit gene (COLQ): How does G at position +3 result in aberrant splicing? Am. J. Hum. Genet. 65, 635–644.
Shapira Y. A., Sadeh M. E., Bergtraum M. P., Tsujino A., Ohno K., Shen X.-M., Brengman J. M., Edwardson S., Matoh I., and Engel A. G. (2002) The novel COLQ mutations and variation of phenotypic expressivity due to G240X. Neurology 58, 603–609.
Karlin A., and Akabas M. H. (1994) Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron 15, 1231–1244.
Prince R. J., and Sine S. M. (1998) The ligand binding domains of the nicotinic acetylcholine receptor, in The Nicotinic Acetylcholine Receptor: Current Views and Future Trends (Barrantes F. J., eds.) Landes Bioscience, Austin, TX, pp. 31–59.
Corringer J. P., Le Novère N., and Changeux J.-P. (2000) Nicotine receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 40, 431–458.
Galzi J.-L., Revah F., Black D., Goeldner M., Hirth C., and Changeux J.-P. (1990) Identification of a novel amino acid α-tyrosine 93 within the cholinergic ligand-binding sites of the acetylcholine receptor by photoaffinity labeling. J. Biol. Chem. 265, 10,430–10,437.
Czajkowski C., and Karlin A. (1995) Structure of the nicotinic receptor acetylcholine binding site. J. Biol. Chem. 270, 3160–3164.
Chiara D. C., Middleton R. E., and Cohen J. B. (1998) Identification of tryptophan 55 as the primary site of [3H]nicotine photoincorporation in the gamma subunit of Torpedo nicotinic acetylcholine receptor. FEBS Lett. 423, 223–226.
Wang D., Chirar D. C., Xie Y., and Cohen J. B. (2000) Probing the structure of the nicotinic acetylcholine receptor with 4-benzoylbenzoyl choline, a novel photoaffinity competitive antagonist. J. Biol. Chem. 275, 28,666–28,674.
Brejc K., van Dijk W. V., Schuurmans M., van der Oost J., Smit A. B., and Sixma T. K. (2001) Crystal structure of ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276.
Miyazawa A., Fujiyoshy Y., and Unwin N. (1999) Nicotinic acetylcholine receptor at 4.6Å resolution: Transverse tunnels in the channel wall. J. Mol. Biol. 288, 765–786.
Unwin N. (1995) Acetylcholine receptor channel imaged in the open state. Nature 373, 37–43.
Akabas M. H., Kaufmann C., Archdeacon P., and Karlin A. (1994) Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the alpha subunit. Neuron 13, 919–927.
Corringer J. P., Bertrand S., Galzi J.-L., Devillers-Thiéry A., Changeux J.-P., and Bertrand D. (1999) Mutational analysis of the charge selectivity filter of the α7 nicotinic acetylcholine receptor. Neuron 22, 831–843.
Milone M., Wang H.-L., Ohno K., Prince R. J., Shen X.-M., Brengman J. M., Griggs R. C., and Engel A. G. (1998) Mode switching kinetics produced by a naturally occurring mutation in the cytoplasmic loop of the human acetylcholine receptor ɛ subunit. Neuron 20, 575–588.
Ohno K., Hutchinson D. O., Milone M., Brengman J. M., Bouzat C., Sine S. M., and Engel A. G. (1995) Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the ɛ subunit. Proc. Natl. Acad. Sci. USA. 92, 758–762.
Sine S. M., Ohno K., Buzat C., Auerbach A., Milone M., Pruitt J. N., and Engel A. G. (1995) Mutation of the acetylcholine receptor α subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron 15, 229–239.
Engel A. G., Ohno K., Milone M., Wang H.-L., Nakano S., Bouzat C., Pruitt J. N., Hutchinson D. O., Brengman J. M., Bren N., Sieb J. P., and Sine S. M. (1996) New mutations in acetylcholine receptor subunit genes reveal heterogeneity in the slow-channel congenital myasthenic syndrome. Hum. Mol. Genet. 5, 1217–1227.
Wang H.-L., Auerbach A., Bren N., Ohno K., Engel A. G., and Sine S. M. (1997) Mutation in the M1 domain of the acetylcholine receptor alpha subunit decreases the rate of agonist dissociation. J. Gen. Physiol. 109, 757–766.
Milone M., Wang H.-L., Ohno K., Fukudome T., Pruitt J. N., Bren N., Sine S. M., and Engel A. G. (1997) Slow-channel syndrome caused by enhanced activation, desensitization, and agonist binding affinity due to mutation in the M2 domain of the acetylcholine receptor alpha subunit. J. Neurosci. 17, 5651–5665.
Gomez C. M., Maselli R., Gammack J., Lasalde J., Tamamizu s., Cornblath D. R., Lehar M., McNamee M., and Kuncl R. (1996) A beta-subunit mutation in the acetylcholine receptor gate causes severe slow-channel syndrome. Ann. Neurol. 39, 712–723.
Croxen R., Newland C., Beeson D., Oosterhuis H., Chauplanaz G., Vincent A., and Newsom-Davis J. (1997) Mutations in different functional domains of the human muscle acetylcholine receptor α subunit in patients with the slow-channel congenital myasthenic syndrome. Hum. Mol. Genet. 6, 767–774.
Ohno K., Milone M., Brengman J. M., Lo Monaco M., Evoli A., Tonali P., and Engel A. G. (1998) Slow-channel congenital myasthenic syndrome caused by a novel mutation in the acetylcholine receptor ɛ subunit. (Abstract) Neurology 50, A432.
Ohno K., Wang H.-L., Shen X.-M., Milone M., Bernasconi L., Sine S. M., and Engel A. G. (2000) Slow-channel mutations in the center of the M1 transmembrane domain of the acetylcholine receptor α subunit. (Abstract) Neurology 54(Suppl 3), A183.
Gomez C. M., Maselli R., Staub J., Day J. W., Cens T., Wollman R. L., and Charnet P. C. (1998) Novel δ and β subunit acetylcholine receptor mutations in the slow-channel syndrome demonstrate phenotypic variability. (Abstract) Soc. Neurosci. Abstr. 24, 484.
Grosman C., Salamone F. N., Sine S. M., and Auerbach A. (2000) The extracellular linker of muscle acetylcholine receptor channels is a gating control element. J. Gen. Physiol. 116, 327–339.
Monod J., Wyman J., and Changeux J.-P. (1965) On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12, 88–118.
Jackson M. B. (1989) Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc. Natl. Acad. Sci. USA 86, 2199–2203.
Labarca C., Nowak M. W., Zhang H., Tang L., Desphpande P., and Lester H. A. (1995) Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature 376, 514–516.
Dennis M., Giraudat J., Kotzyba-Hilbert F., Goeldner M., Hirth C., Chang J., Lazure C., and Cretien M. (1988) Amino acids of Torpedo marmotrata acetylcholine receptor alpha subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry. 27, 2346–2357.
Ohno K., Wang H.-L., Milone M., Bren N., Brengman J. M., Nakano S., Quiram P., Pruitt J. N., Sine S. M., and Engel A. G. (1996) Congenital myasthenic syndrome caused by decreased agonist binding affinity due to a mutation in the acetylcholine receptor ɛ subunit. Neuron 17, 157–170.
Shen X.-M., Ohno K., Milone M., Brengman, J. M., Spilsbury P. R., and Engel A. G. (2001) Fast-channel syndrome. (Abstract) Neurology 56(suppl. 3), A60.
Shen X.-M., Tsujino A., Ohno K., Brengman J. M., Gingold M., and Engel A. G. (2000) A novel fast-channel congenital myasthenic syndrome caused by a mutation in the Cys-loop domain of the acetylcholine receptor ɛ subunit. (Abstract) Neurology 54(suppl 3), A138.
Wang H.-L., Milone M., Ohno K., Shen X.-M., Tsujino A., Batocchi A. P., Tonali P., Brengman J. M., Engel A. G., and Sine S. M. (1999) Acetylcholine receptor M3 domain: Stereochemical and volume contributions to channel gating. Nature Neurosci. 2, 226–233.
Wang H.-L., Ohno K., Milone M., Brengman J. M., Evoli A., Batocchi A. P., Middleton L., Christodoulou K., Engel A. G., and Sine S. M. (2000) Fundamental gating mechanism of nicotinic receptor channel revealed by mutation causing a congenital myasthenic syndrome. J. Gen. Physiol. 116, 449–460.
Shen X.-M., Ohno K., Fukudome T., Brengman J. M., and Engel A. G. (1999) Deletion of a single codon from the long cytoplasmic loop of the nAChR subunit gene causes brief single channel currents. (Abstract) Soc. Neurosci. Abstr. 25, 1721.
Brownlow S., Webster R., Croxen R., Brydson M., Neville B., Lin J.-P., Vincent A., Newsom-Davis J., and Beeson D. (2001) Acetylcholine receptor δ subunit mutations underlie a fast-channel myasthenic syndrome and arthrogryposis multiplex congenita. J. Clin. Invest. 108, 125–130.
Engel A. G., Ohno K., Bouzat C., Sine S. M., and Griggs R. G. (1996) End-plate acetylcholine receptor deficiency due to nonsense mutations in the ɛ subunit. Ann. Neurol. 40, 810–817.
Ohno K., Quiram P., Milone M., Wang H.-L., Harper C. M., Pruitt J. N., Brengman J. M., Pao L., Fischbeck K. H., Crawford T. O., Sine S. M., and Engel A. G. (1997) Congenital myasthenic syndromes due to heteroallelic nonsense/missense mutations in the acetylcholine receptor ɛ subunit gene: identification and functional characterization of six new mutations. Hum. Mol. Genet. 6, 753–766.
Ohno K., Engel A. G., Milone M., Brengman J. M., Sieb J. P., and Iannaccone S. (1995) A congenital myasthenic syndrome with severe acetylcholine receptor deficiency caused by heteroallelic frameshifting mutations in the epsilon subunit. (Abstract) Neurology 45(Suppl 4), A283.
Ohno K., Anlar B., Özdirim E., Brengman J. M., De Bleecker J., and Engel A. G. (1998) Myasthenic syndromes in Turkish kinships due to mutations in the acetylcholine receptor. Ann. Neurol. 44, 234–241.
Ohno K., Fukudome T., Nakano S., Milone M., Feasby T. E., Tyce G. M., and Engel A. G. (1996) Mutational analysis in a congenital myasthenic syndrome reveals a novel acetylcholine receptor epsilon subunit mutation. Soc. Neurosci. Abstr. 22, 234–234.
Middleton L., Ohno K., Christodoulou K., et al. (1999) Congenital myasthenic syndromes linked to chromosome 17p are caused by defects in acetylcholine receptor ɛ subunit gene. Neurology 53, 1076–1082.
Croxen R., Beeson D., Vincent A., and Newsom-Davis J. (1996) Congenital myasthenic syndrome with a single nucleotide deletion at the intron/exon boundary in exon 12 of the gene encoding the acetylcholine receptor ɛ subunit. (Abstract) Ann. Neurol. 40, 513.
Croxen R., Newland C., Betty M., Vincent A., Newsom-Davis J., and Beeson D. (1999) Novel functional ɛ-subunit polypeptide generated by a single nucleotide deletion in acetylcholine receptor deficiency congenital myasthenic syndrome. Ann. Neurol. 46, 639–647.
Abicht A., Stucka R., Karcagi V., et al. (1999) A common mutation (ɛ1267delG) in congenital myasthenic patients of Gipsy ethnic origin. Neurology 53, 1564–1569.
Ohno K., Anlar B., and Engel A. G. (1999) Congenital myasthenic syndrome caused by a mutation in the Ets-binding site of the promoter region of the acetylcholine receptor ɛ subunit gene. Neuromuscul. Disord. 9, 131–135.
Nichols P., Croxen R., Vincent A., Rutter R., Hutchinson M., Newsom-Davis J., and Beeson D. (1999) Mutation of the acetylcholine receptor ɛ-subunit promoter in congenital myasthenic syndrome. Ann. Neurol. 45, 439–443.
Quiram P., Ohno K., Milone M., Patterson M. C., Pruitt N. J., Brengman J. M., Sine S. M., and Engel A. G. (1999) Mutation causing congenital myasthenia reveals acetylcholine receptor β/δ subunit interaction essential for assembly. J. Clin. Invest. 104, 1403–1410.
Ohno K., and Engel A. G. (2002) Congenital myasthenic syndromes: gene mutations. Neuromuscul. Disord. (In press).
Gautam M., Noakes P. G., Moscoso L., Rupp F., Scheller R. H., Merlie M. P., and Sanes J. R. (1996) Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535.
Burgess R. W., Nguyen Q. T., Son Y. J., Lichtman J. W., and Sanes J. R. (1999) Alternatively spliced isoforms of nerve- and muscle-derived agrin: Their roles at the neuromuscular junction. Neuron 23, 33–44.
Glass D. J., Bowen D. C., Stitt T. N., et al. (1996) Agrin acts via MuSK receptor complex. Cell 85, 513–523.
Apel E. D., Glass D. J., Moscosco L. M., Yancopoulos G. D., and Sanes J. R. (1997) Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18, 623–625.
Smith C. L., Mittaud P., Prescott E. D., Fuhrer C., and Burden S. J. (2001) Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrininduced clusters of acetylcholine receptors. J. Neurosci. 21, 3151–3160.
Cartaud A., Coutant S., Petrucci T. C., and Cartaud J. (1998) Evidence for in situ and in vitro association between β-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J. Biol. Chem. 273, 11,321–11,326.
Tseng C. N., Yao Y., Wang J. M., Viroonchatapan N., Rothe E., and Wang Z. Z. (2001) A synaptic isoform of NRAP interacts with the postsynaptic 43K protein rapsyn and links it to the cytoskeleton at the neuromuscular junction. Soc. Neurosci. Abstr. 27, Program No. 694.6.
Sandrock A. W., Dryer S. E., Rosen K. M., Gozani S. M., Kramer R., Theill L. E., and Fischbach G. D. (1997) Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 276, 599–603.
Si J., Luo Z., and Mei L. (1996) Induction of acetylcholine receptor gene expression by ARIA requires activation of mitogen-activated protein kinase. J. Biol. Chem. 271, 19,752–19,759.
Altiok N., Altiok K., and Changeux J.-P. (1997) Heregulin-stimulated acetylcholine receptor gene expression in muscle — requirement for MAP kinase and evidence for parallel inhibitory pathway independent electrical activity. EMBO J. 16, 717–725.
Belluardo N., Westerblad H., Mudo G., Casabona A., Bruton J., Caniglia G., Pastoris O., Grassi F., and Ibanez C. F. (2001) Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Moll. Cell. Neurosci. 18, 56–67.
Gonzales M., Ruggiero F. P., Chang Q., Shi Y. J., Rich M. M., Kraner S., and Balice-Gordon R. J. (1999) Disruption of Trkb-mediated signaling induces disassembly of potsynaptic receptor clusters at neuromuscular junctions. Neuron 24, 567–583.
Newey S. A., Gramolini A. O., Wu J., Izfeind G., Smin B. J., Vies K. E., and Ake D. J. (2001) A novel mechanism for modulating synaptic gene expression: Differential localization of α-dystrobrevin transcripts in skeletal muscle. Moll. Cell. Neurosci. 17, 127–140.
Grady R. M., Merlie J. P., and Sanes J. R. (1997) Subtle neuromuscular defects in utrophin-deficient mice. J. Cell. Biol. 136, 871–882.
Adams M. E., Kramarcy M., Krall S. P., Rossi S. G., Rotundo R. L., Sealock R., and Froehner S. C. (2000) Absence of α-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J. Cell Biol. 150, 1385–1398.
Banwell B. L., Russel J., Fukudome T., Shen X.-M., Stilling G., and Engel A. G. (1999) Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency. J. Neuropathol. Exp. Neurol. 58, 832–846.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engel, A.G., Ohno, K. & Sine, S.M. The spectrum of congenital myasthenic syndromes. Mol Neurobiol 26, 347–367 (2002). https://doi.org/10.1385/MN:26:2-3:347
Issue Date:
DOI: https://doi.org/10.1385/MN:26:2-3:347