Abstract
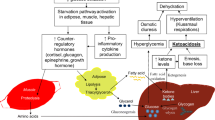
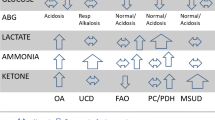
Congenital hyperinsulinism (CHI) is a clinically and genetically heterogeneous entity and causes severe hypoglycemia in neonates and infants. The clinical heterogeneity is manifested by severity ranging from extremely severe, life-threatening disease to very mild clinical symptoms, which may even be difficult to identify. Furthermore, clinical responsiveness to medical and surgical management is extremely variable.
Recent discoveries have begun to clarify the molecular etiology of this disease in about 50% of cases. Mutations in five different genes have been identified in patients with this clinical syndrome. Most cases are caused by mutations in the genes ABCC8 and KCNJ11 coding for either of the two subunits of the beta-cell KATP channel (SUR1 and Kir6.2). Recessive mutations of the beta-cell K(ATP) channel genes cause diffuse HI, whereas loss of heterozygosity together with inheritance of a paternal mutation causes focal adenomatous HI. In other cases, CHI is caused by mutations in genes coding for the beta-cell enzymes glucokinase (GK), glutamate dehydrogenase (GDH), and SCHAD.
However, for as many as 50% of the cases, no genetic etiology has yet been determined. The study of the genetics of this disease has provided important new information regarding beta-cell physiology.
Similar content being viewed by others
References
de Lonlay P, Fournet JC, Touati G, et al. Heterogeneity of persistent hyperinsulinaemic hypoglycaemia. A series of 175 cases. Eur J Pediatr 161:37–48, 2002.
Rahier J, Sempoux C, Fournet JC, et al. Partial or near-total pancreatectomy for persistent neonatal hyperinsulinaemic hypoglycaemia: the pathologist’s role. Histopathology 32:15–19, 1998.
Dunne MJ, Cosgrove KE, Shepherd RM, Ammala C. Potassium channels, sulphonylurea receptors and control of insulin release. Trends Endocrinol Metab 10:146–152, 1999.
Thameem F, Farook VS, Yang X, et al. The transcribed endosulfine alpha gene is located within a type 2 diabetes-linked region on 1q: sequence and expression analysis in Pima Indians. Mol Genet Metab 81:16–21, 2004.
Wang H, Craig RL, Schay J, et al. Alphaendosulfine, a positional and functional candidate gene for type 2 diabetes: molecular screening, association studies, and role in reduced insulin secretion. Mol Genet Metab; 81:9–15, 2004.
Stanley CA. Hyperinsulinism in infants and children. Pediatr Clin North Am 44:363–374, 1997.
Yap F, Hogler W, Vora A, Halliday R, Ambler G. Severe transient hyperinsulinaemic hypoglycaemia: two neonates without predisposing factors and a review of the literature. Eur J Pediatr 163:38–41, 2004.
Nestorowicz A, Glaser B, Wilson BA, et al. Genetic heterogeneity in familial hyperinsulinism. Hum Mol Genet 7:1119–1128, 1998.
Glaser B, Thornton P, Otonkoski T, Junien C. Genetics of neonatal hyperinsulinism. Arch Dis Child Fetal Neonatal Ed 82:F79–86, 2000.
Shepherd RM, Cosgrove KE, O’Brien RE, Barnes PD, Ammala C, Dunne MJ. Hyperinsulinism of infancy: towards an understanding of unregulated insulin release. European Network for Research into Hyperinsulinism in Infancy. Arch Dis Child Fetal Neonatal Ed 82:F87–97, 2000.
Nestorowicz A, Wilson BA, Schoor KP, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet 5:1813–1822, 1996.
Otonkoski T, Ammala C, Huopio H, et al. A point mutation inactivating the sulfonylurea receptor causes the severe form of persistent hyperinsulinemic hypoglycemia of infancy in Finland. Diabetes 48:408–415, 1999.
Darendeliler F, Fournet JC, Bas F, et al. ABCC8 (SUR1) and KCNJ11 (KIR6.2) mutations in persistent hyperinsulinemic hypoglycemia of infancy and evaluation of different therapeutic measures. J Pediatr Endocrinol Metab 15:993–1000, 2002.
Huopio H, Reimann F, Ashfield R, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest 106:897–906, 2000.
Thornton PS, MacMullen C, Ganguly A, et al. Clinical and molecular characterization of a dominant form of congenital hyperinsulinism caused by a mutation in the high-affinity sulfonylurea receptor. Diabetes 52:2403–2410, 2003.
Dekel B, Lubin D, Modan-Moses D, Quint J, Glaser B, Meyerovitch J. Compound het-erozygosity for the common sulfonylurea receptor mutations can cause mild diazoxide-sensitive hyperinsulinism. Clin Pediatr (Phila) 41:183–186, 2002.
Suchi M, MacMullen C, Thornton PS, Ganguly A, Stanley CA, ruchelli ED. Histopathology of congenital hyperinsulinism: retrospective study with genotype correlations. Pediatr Dev Pathol 6:322–333, 2003.
Verkarre V, Fournet JC, de Lonlay P, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest 102:1286–1291, 1998.
de Lonlay P, Fournet JC, Rahier J, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest 100:802–807, 1997.
Fournet JC, Mayaud C, de Lonlay P, et al. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: association with a reduction to homozygosity of a mutation in ABCC8 or KCNJ11. Am J Pathol 158:2177–2184, 2001.
Kassem SA, Ariel I, Thornton PS, et al. p57(KIP2) expression in normal islet cells and in hyperinsulinism of infancy. Diabetes 50:2763–2769, 2001.
Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49:1325–1333, 2000.
Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 338: 1352–1357, 1998.
Stanley CA. Hyperinsulinism/hyperammonemia syndrome: insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol Genet Metab 81(Suppl):45–51, 2004.
Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med 338: 226–230, 1998.
Dullaart RP, Hoogenberg K, Rouwe CW, Stulp BK. Family with autosomal dominant hyperinsulinism associated with A456V mutation in the glucokinase gene. J Intern Med 255:143–145, 2004.
Molven A, Matre GE, Duran M, et al. Familial hyperinsulinemic hypoglycermia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes 53:221–227, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fournet, JC., Junien, C. Genetics of congenital hyperinsulinism. Endocr Pathol 15, 233–239 (2004). https://doi.org/10.1385/EP:15:3:233
Issue Date:
DOI: https://doi.org/10.1385/EP:15:3:233