Key Points
-
The current notion that obesity is a major risk factor for development of, and mortality associated with a subset of cancers is well appreciated; however, detailed mechanistic insights underlying this relationship are still lacking
-
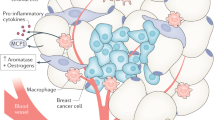
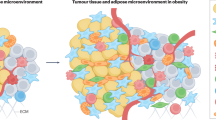
Adipose tissues that influence functions of cancer cells, such as growth, metastasis and recurrence, are an integral part of the tumour microenvironment
-
Heterotypic signals between cancer-associated adipocytes and cancer cells provide a permissive niche for the growth and metastasis of tumours
-
Obesity-related inflammation is a plausible link between obesity and cancer
-
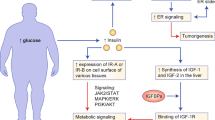
Metabolic symbiosis between stromal adipocytes and cancer cells may account, in part, for the link between obesity and cancer
-
The effects of obesity on cancer progression might be specifically curbed through weight-loss interventions, such as exercise or medication
Abstract
Over the past several years, the field of cancer research has directed increased interest towards subsets of obesity-associated tumours, which include mammary, renal, oesophageal, gastrointestinal and reproductive cancers in both men and women. The increased risk of breast cancer that is associated with obesity has been widely reported; this has drawn much attention and as such, warrants investigation of the key mechanisms that link the obese state with cancer aetiology. For instance, the obese setting provides a unique adipose tissue microenvironment with concomitant systemic endocrine alterations that favour both tumour initiation and progression. Major metabolic differences exist within tumours that distinguish them from non-transformed healthy tissues. Importantly, considerable metabolic differences are induced by tumour cells in the stromal vascular fraction that surrounds them. The precise mechanisms that underlie the association of obesity with cancer and the accompanying metabolic changes that occur in the surrounding microenvironment remain elusive. Nonetheless, specific therapeutic agents designed for patients with obesity who develop tumours are clearly needed. This Review discusses recent advances in understanding the contributions of obesity to cancer and their implications for tumour treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Unger, R. H. & Scherer, P. E. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol. Metab. 21, 345–352 (2010).
Parekh, N., Chandran, U. & Bandera, E. V. Obesity in cancer survival. Annu. Rev. Nutr. 32, 311–342 (2012).
Park, J., Euhus, D. M. & Scherer, P. E. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr. Rev. 32, 550–570 (2011).
Barlow, W. E. et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J. Natl Cancer Inst. 98, 1204–1214 (2006).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348, 1625–1638 (2003).
Zhang, Y. et al. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 72, 5198–5208 (2012).
Zheng, Q. et al. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr. Relat. Cancer 20, 797–808 (2013).
Park, E. J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Campbell, P. T. et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study–II Nutrition Cohort. J. Clin. Oncol. 30, 42–52 (2012).
Bastarrachea, J., Hortobagyi, G. N., Smith, T. L., Kau, S. W. & Buzdar, A. U. Obesity as an adverse prognostic factor for patients receiving adjuvant chemotherapy for breast cancer. Ann. Intern. Med. 120, 18–25 (1994).
Meyerhardt, J. A. et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J. Clin. Oncol. 22, 648–657 (2004).
Semenkovich, C. F. Insulin resistance and atherosclerosis. J. Clin. Invest. 116, 1813–1822 (2006).
Berg, A. H. & Scherer, P. E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 96, 939–949 (2005).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Ostman, A. & Augsten, M. Cancer-associated fibroblasts and tumor growth—bystanders turning into key players. Curr. Opin. Genet. Dev. 19, 67–73 (2009).
Lewis, C. E. & Pollard, J. W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 66, 605–612 (2006).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Tessitore, L. et al. Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int. J. Oncol. 24, 1529–1535 (2004).
Park, J. & Scherer, P. E. Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Invest. 122, 4243–4256 (2012).
Nieman, K. M., Romero, I. L., Van Houten, B. & Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 1831, 1533–1541 (2013).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011).
Harris, A. L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 (2002).
Rutkowski, J. M., Davis, K. E. & Scherer, P. E. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 276, 5738–5746 (2009).
Halberg, N. et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 29, 4467–4483 (2009).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell Biol. 29, 1575–1591 (2009).
Sun, K., Tordjman, J., Clement, K. & Scherer, P. E. Fibrosis and adipose tissue dysfunction. Cell. Metab. 18, 470–477 (2013).
Park, J. & Scherer, P. E. Endotrophin—a novel factor linking obesity with aggressive tumor growth. Oncotarget 3, 1487–1488 (2012).
Iyengar, P. et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 115, 1163–1176 (2005).
Iyengar, P. et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene 22, 6408–6423 (2003).
Sun, K., Halberg, N., Khan, M., Magalang, U. J. & Scherer, P. E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell Biol. 33, 904–917 (2013).
Kusminski, C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012).
Neels, J. G., Thinnes, T. & Loskutoff, D. J. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 18, 983–985 (2004).
Brown, J. M. & McIntosh, M. K. Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J. Nutr. 133, 3041–3046 (2003).
Naugler, W. E. & Karin, M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 14, 109–119 (2008).
Andarawewa, K. L. et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell–adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 65, 10862–10871 (2005).
Dirat, B. et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71, 2455–2465 (2011).
Wyckoff, J. et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 64, 7022–7029 (2004).
Zhang, X. H. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 (2013).
Cufi, S. et al. Metformin-induced preferential killing of breast cancer initiating CD44+CD24−/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts. Oncotarget 3, 395–398 (2012).
Singh, A. & Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751 (2010).
Frank, N. Y., Schatton, T. & Frank, M. H. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 120, 41–50 (2010).
Korkaya, H. et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell 47, 570–584 (2012).
Hinohara, K. & Gotoh, N. Inflammatory signaling pathways in self-renewing breast cancer stem cells. Curr. Opin. Pharmacol. 10, 650–654 (2010).
Asiedu, M. K., Ingle, J. N., Behrens, M. D., Radisky, D. C. & Knutson, K. L. TGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 71, 4707–4719 (2011).
Scheel, C. et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145, 926–940 (2011).
Zheng, Q. et al. Leptin deficiency suppresses MMTV–Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr. Relat. Cancer 18, 491–503 (2011).
Feldman, D. E., Chen, C., Punj, V., Tsukamoto, H. & Machida, K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc. Natl Acad. Sci. USA 109, 829–834 (2012).
Park, J., Morley, T. S. & Scherer, P. E. Inhibition of endotrophin, a cleavage product of collagen VI, confers cisplatin sensitivity to tumours. EMBO Mol. Med. 5, 935–948 (2013).
Park, H. S., Park, J. Y. & Yu, R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 69, 29–35 (2005).
He, G. et al. Identification of liver cancer progenitors whose malignant progression depends on autocrine IL-6 signaling. Cell 155, 384–396 (2013).
Flores, M. B. et al. Obesity-induced increase in tumor necrosis factor-α leads to development of colon cancer in mice. Gastroenterology 143, 741–753. e1–e4 (2012).
Hill-Baskin, A. E. et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum. Mol. Genet. 18, 2975–2988 (2009).
Shimizu, M. et al. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J-+(db)/+Lepr(db) mice. Cancer Prev. Res. (Phila.) 4, 128–36 (2011).
Yehuda-Shnaidman, E. & Schwartz, B. Mechanisms linking obesity, inflammation and altered metabolism to colon carcinogenesis. Obes. Rev. 13, 1083–1095 (2012).
Jain, S. S. & Bird, R. P. Elevated expression of tumor necrosis factor-α signaling molecules in colonic tumors of Zucker obese (fa/fa) rats. Int. J. Cancer 127, 2042–2050 (2010).
Teraoka, N. et al. High susceptibility to azoxymethane-induced colorectal carcinogenesis in obese KK-Ay mice. Int. J. Cancer 129, 528–535 (2011).
Mentor-Marcel, R. A. et al. Inflammation-associated serum and colon markers as indicators of dietary attenuation of colon carcinogenesis in ob/ob mice. Cancer Prev. Res. (Phila.) 2, 60–69 (2009).
Yasuda, Y. et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci. 101, 1701–7 (2010).
Kubota, M. et al. Renin–angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun. 410, 108–113 (2011).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Liu, Z. et al. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J. Nutr. Biochem. 23, 1207–1213 (2012).
Chia, V. M. et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev. 16, 2697–2703 (2007).
Aleksandrova, K. et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 72, 5328–5337 (2012).
Wang, D. et al. Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J. Biosci. 37, 91–101 (2012).
Ogunwobi, O. O. & Beales, I. L. The anti-apoptotic and growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int. J. Colorectal Dis. 22, 401–409 (2007).
Endo, H. et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 60, 1363–1371 (2011).
Uddin, S. et al. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol. Cancer 8, 74 (2009).
Chen, C., Chang, Y. C., Lan, M. S. & Breslin, M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int. J. Oncol. 42, 1113–1119 (2013).
Ptak, A., Kolaczkowska, E. & Gregoraszczuk, E. L. Leptin stimulation of cell cycle and inhibition of apoptosis gene and protein expression in OVCAR-3 ovarian cancer cells. Endocrine 43, 394–403 (2013).
Ptak, A. & Gregoraszczuk, E. L. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat., MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol. Lett. 210, 332–337 (2012).
Ptak, A., Rak-Mardyla, A. & Gregoraszczuk, E. L. Cooperation of bisphenol A and leptin in inhibition of caspase-3 expression and activity in OVCAR-3 ovarian cancer cells. Toxicol. In Vitro 27, 1937–1943 (2013).
Niu, J. et al. The association between leptin level and breast cancer: a meta-analysis. PLoS ONE 8, e67349 (2013).
Miyoshi, Y. et al. High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int. J. Cancer 118, 1414–1419 (2006).
Cirillo, D., Rachiglio, A. M., la Montagna, R., Giordano, A. & Normanno, N. Leptin signaling in breast cancer: an overview. J. Cell Biochem. 105, 956–964 (2008).
Andó, S. & Catalano, S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat. Rev. Endocrinol. 8, 263–275 (2012).
Park, J., Kusminski, C. M., Chua, S. C. & Scherer, P. E. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am. J. Pathol. 177, 3133–3144 (2010).
Holland, W. L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011).
Kamada, Y. et al. Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J. Hepatol. 47, 556–564 (2007).
Saxena, N. K. et al. Adiponectin modulates C-jun N-terminal kinase and mammalian target of rapamycin and inhibits hepatocellular carcinoma. Gastroenterology 139, 1762–1773 (2010).
Man, K. et al. Suppression of liver tumor growth and metastasis by adiponectin in nude mice through inhibition of tumor angiogenesis and downregulation of Rho kinase/IFN-inducible protein 10/matrix metalloproteinase 9 signaling. Clin. Cancer Res. 16, 967–977 (2010).
Sharma, D. et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 52, 1713–1722 (2010).
Wei, E. K., Giovannucci, E., Fuchs, C. S., Willett, W. C. & Mantzoros, C. S. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J. Natl Cancer Inst. 97, 1688–1694 (2005).
Yoneda, K. et al. Expression of adiponectin receptors, AdipoR1 and AdipoR2, in normal colon epithelium and colon cancer tissue. Oncol. Rep. 20, 479–483 (2008).
Kim, A. Y. et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol. Endocrinol. 24, 1441–1452 (2010).
Sugiyama, M. et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 34, 339–344 (2009).
Mutoh, M. et al. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology 140, 2000–2008 (2011).
Fujisawa, T. et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 57, 1531–1538 (2008).
Tworoger, S. S. et al. Plasma adiponectin concentrations and risk of incident breast cancer. J. Clin. Endocrinol. Metab. 92, 1510–1516 (2007).
Denzel, M. S. et al. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin. Cancer Res. 15, 3256–3264 (2009).
Landskroner-Eiger, S. et al. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin. Cancer Res. 15, 3265–3276 (2009).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011).
Pavlides, S. et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8, 3984–4001 (2009).
Migneco, G. et al. Glycolytic cancer associated fibroblasts promote breast cancer tumor growth, without a measurable increase in angiogenesis: evidence for stromal–epithelial metabolic coupling. Cell Cycle 9, 2412–2422 (2010).
Gallagher, E. J. & LeRoith, D. Diabetes, antihyperglycemic medications and cancer risk: smoke or fire? Curr. Opin. Endocrinol. Diabetes Obes. 20, 485–494 (2013).
Brand-Miller, J. C. Glycemic load and chronic disease. Nutr. Rev. 61, S49–S55 (2003).
Vigneri, P., Frasca, F., Sciacca, L., Pandini, G. & Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 16, 1103–1123 (2009).
Ferguson, R. D., Gallagher, E. J., Scheinman, E. J., Damouni, R. & LeRoith, D. The epidemiology and molecular mechanisms linking obesity, diabetes, and cancer. Vitam. Horm. 93, 51–98 (2013).
Warburg, O. The reaction of ascites tumor cells to oxygen under high pressure. Arch. Geschwulstforsch. 6, 7–11 (1953).
Shapot, V. S. & Blinov, V. A. Blood glucose levels and gluconeogenesis in animals bearing transplantable tumors. Cancer Res. 34, 1827–1832 (1974).
Pavelic, K. et al. Growth and treatment of Ehrlich tumor in mice with alloxan-induced diabetes. Cancer Res. 39, 1807–1813 (1979).
Gullino, P. M., Grantham, F. H. & Courtney, A. H. Glucose consumption by transplanted tumors in vivo. Cancer Res. 27, 1031–1040 (1967).
Goranson, E. S. & Tilser, G. J. Studies on the relationship of alloxan-diabetes and tumor growth. Cancer Res. 15, 626–631 (1955).
Park, J., Sarode, V. R., Euhus, D., Kittler, R. & Scherer, P. E. Neuregulin 1–HER axis as a key mediator of hyperglycemic memory effects in breast cancer. Proc. Natl Acad. Sci. USA 109, 21058–21063 (2012).
Wang, Z. V. et al. PANIC-ATTAC: a mouse model for inducible and reversible β-cell ablation. Diabetes 57, 2137–2148 (2008).
Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 8, 915–928 (2008).
Clayton, P. E., Banerjee, I., Murray, P. G. & Renehan, A. G. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 7, 11–24 (2011).
Renehan, A. G., Frystyk, J. & Flyvbjerg, A. Obesity and cancer risk: the role of the insulin–IGF axis. Trends Endocrinol. Metab. 17, 328–336 (2006).
Renehan, A. G. et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363, 1346–1353 (2004).
Parekh, N. et al. Lifestyle, anthropometric, and obesity-related physiologic determinants of insulin-like growth factor-1 in the Third National Health and Nutrition Examination Survey (1988–1994). Ann. Epidemiol. 20, 182–193 (2010).
Crowe, F. L. et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Hum. Biol. 38, 194–202 (2011).
Fogarty, A. W. et al. A prospective study of weight change and systemic inflammation over 9 y. Am. J. Clin. Nutr. 87, 30–35 (2008).
Huang, X. F. & Chen, J. Z. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 10, 610–616 (2009).
O'Brien, K. D. et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid A and C-reactive protein independent of dietary macronutrient composition in obese subjects. J. Clin. Endocrinol. Metab. 90, 2244–2249 (2005).
Tran, C. D., Diorio, C., Berube, S., Pollak, M. & Brisson, J. Relation of insulin-like growth factor (IGF) I and IGF-binding protein 3 concentrations with intakes of fruit, vegetables, and antioxidants. Am. J. Clin. Nutr. 84, 1518–1526 (2006).
Heald, A. H. et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia 44, 333–339 (2001).
Bol, D. K., Kiguchi, K., Gimenez-Conti, I., Rupp, T. & DiGiovanni, J. Overexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic mice. Oncogene 14, 1725–1734 (1997).
DiGiovanni, J. et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc. Natl Acad. Sci. USA 97, 3455–3460 (2000).
Carboni, J. M. et al. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 65, 3781–3787 (2005).
Moorehead, R. A., Sanchez, O. H., Baldwin, R. M. & Khokha, R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene 22, 853–857 (2003).
Pravtcheva, D. D. & Wise, T. L. Metastasizing mammary carcinomas in H19 enhancers Igf2 transgenic mice. J. Exp. Zool. 281, 43–57 (1998).
Santen, R. J., Brodie, H., Simpson, E. R., Siiteri, P. K. & Brodie, A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr. Rev. 30, 343–375 (2009).
Simpson, E. R. et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 15, 342–355 (1994).
Chen, J. Multiple signal pathways in obesity-associated cancer. Obes. Rev. 12, 1063–1070 (2011).
Bulun, S. E., Chen, D., Moy, I., Brooks, D. C. & Zhao, H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol. Metab. 23, 83–89 (2012).
Key, T. et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl Cancer Inst. 94, 606–616 (2002).
Bulun, S. E., Price, T. M., Aitken, J., Mahendroo, M. S. & Simpson, E. R. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J. Clin. Endocrinol. Metab. 77, 1622–1628 (1993).
Oneill, J. S., Elton, R. A. & Miller, W. R. Aromatase activity in adipose tissue from breast quadrants: a link with tumour site. Br. Med. J. 296, 741–743 (1988).
Chen, D. et al. Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res. 67, 8914–8922 (2007).
Zhao, Y., Nichols, J. E., Valdez, R., Mendelson, C. R. & Simpson, E. R. Tumor necrosis factor-α stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol. Endocrinol. 10, 1350–1357 (1996).
Ellis, M. J. et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486, 353–360 (2012).
Jansen, M. P. et al. Hallmarks of aromatase inhibitor drug resistance revealed by epigenetic profiling in breast cancer. Cancer Res. 73, 6632–6641 (2013).
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R. & Morris, A. D. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005).
Li, D., Yeung, S. C., Hassan, M. M., Konopleva, M. & Abbruzzese, J. L. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137, 482–488 (2009).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Zakikhani, M., Dowling, R., Fantus, I. G., Sonenberg, N. & Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 66, 10269–10273 (2006).
Dowling, R. J., Zakikhani, M., Fantus, I. G., Pollak, M. & Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 67, 10804–10812 (2007).
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N. & Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 69, 7507–7511 (2009).
Satoh, T. et al. Activation of peroxisome proliferator-activated receptor-γ stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene 21, 2171–2180 (2002).
Girnun, G. D. et al. Regression of drug-resistant lung cancer by the combination of rosiglitazone and carboplatin. Clin. Cancer Res. 14, 6478–6486 (2008).
Girnun, G. D. et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell 11, 395–406 (2007).
McNeely, M. L. et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ 175, 34–41 (2006).
Holmes, M. D., Chen, W. Y., Feskanich, D., Kroenke, C. H. & Colditz, G. A. Physical activity and survival after breast cancer diagnosis. JAMA 293, 2479–2486 (2005).
Thompson, H. J. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res. 54, 1960s–1963s (1994).
Thompson, H. J., Westerlind, K. C., Snedden, J., Briggs, S. & Singh, M. Exercise intensity dependent inhibition of 1-methyl-1-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis 16, 1783–1786 (1995).
Thompson, H. J., Ronan, A. M., Ritacco, K. A., Tagliaferro, A. R. & Meeker, L. D. Effect of exercise on the induction of mammary carcinogenesis. Cancer Res. 48, 2720–2723 (1988).
Chlebowski, R. T. et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J. Natl Cancer Inst. 98, 1767–1776 (2006).
Visser, M., Bouter, L. M., McQuillan, G. M., Wener, M. H. & Harris, T. B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282, 2131–2135 (1999).
Tchernof, A., Nolan, A., Sites, C. K., Ades, P. A. & Poehlman, E. T. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation 105, 564–569 (2002).
Imayama, I. et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 72, 2314–2326 (2012).
Silha, J. V., Krsek, M., Sucharda, P. & Murphy, L. J. Angiogenic factors are elevated in overweight and obese individuals. Int. J. Obes. (Lond.) 29, 1308–1314 (2005).
Acknowledgements
The authors' research was supported by NIH Grants R01-DK55758, R01-DK099110 (to P.E.S.) and P01-DK088761 (to P.E.S. and D.J.C.). J.P.'s research was supported by a fellowship from the US Department of Defence (USAMRMC BC085909) and 2014 research fund (1.130088.01) of Ulsan National Institute of Science and Technology. T.S.M.'s work was supported by NIH Training Grant T32-GM083831.
Author information
Authors and Affiliations
Contributions
J.P. and T.S.M. contributed equally to this article. J.P., T.S.M., D.J.C. and P.E.S undertook discussions of the article content, writing, and review or editing of the manuscript before submission. In addition, M.K. contributed to discussions of the content and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Park, J., Morley, T., Kim, M. et al. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10, 455–465 (2014). https://doi.org/10.1038/nrendo.2014.94
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.94
This article is cited by
-
MIIP downregulation drives colorectal cancer progression through inducing peri-cancerous adipose tissue browning
Cell & Bioscience (2024)
-
m6A modification mediates SLC3A2/SLC7A5 translation in 3-methylcholanthrene-induced uroepithelial transformation
Cell Biology and Toxicology (2024)
-
Adipose-derived exosomal miR-421 targets CBX7 and promotes metastatic potential in ovarian cancer cells
Journal of Ovarian Research (2023)
-
Adipose tissue macrophages: implications for obesity-associated cancer
Military Medical Research (2023)
-
The role of three-dimensional in vitro models in modelling the inflammatory microenvironment associated with obesity in breast cancer
Breast Cancer Research (2023)