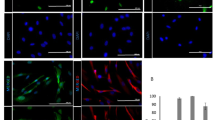
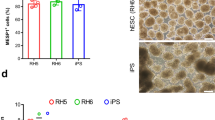
Abstract
Multipotent neural crest stem cells (NCSCs) have the potential to generate a wide range of cell types including melanocytes; peripheral neurons; and smooth muscle, bone, cartilage and fat cells. This protocol describes in detail how to perform a highly efficient, lineage-specific differentiation of human pluripotent cells to a NCSC fate. The approach uses chemically defined media under feeder-free conditions, and it uses two small-molecule compounds to achieve efficient conversion of human pluripotent cells to NCSCs in ∼15 d. After completion of this protocol, NCSCs can be used for numerous applications, including the generation of sufficient cell numbers to perform drug screens, for the development of cell therapeutics on an industrial scale and to provide a robust model for human disease. This protocol can be also be applied to patient-derived induced pluripotent stem cells and thus used to further the knowledge of human disease associated with neural crest development, for example, Treacher-Collins Syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Menendez, L., Yatskievych, T.A., Antin, P.B. & Dalton, S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 108, 19240–19245 (2011).
Shakhova, O. & L., Sommer Neural crest–derived stem cells. in StemBook (ed. the Stem Cell Research Community) doi:10.3824/stembook.1.51.1 (4 May 2010).
Sauka-Spengler, T. & Bronner-Fraser, M. A gene regulatory network orchestrates neural crest formation. Nat. Rev. Mol. Cell Biol. 9, 557–568 (2008).
Le Douarin, N.M. & Dupin, E. Multipotentiality of the neural crest. Curr. Opin. Genet. Dev. 13, 529–536 (2003).
Betancur, P., Bronner-Fraser, M. & Sauka-Spengler, T. Assembling neural crest regulatory circuits into a gene regulatory network. Ann. Rev. Cell Dev. Biol. 26, 581–603 (2010).
Lee, G., Chambers, S.M., Tomishima, M.J. & Studer, L. Derivation of neural crest cells from human pluripotent stem cells. Nat. Protoc. 5, 688–701 (2010).
Patthey, C., Edlund, T. & Gunhaga, L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 136, 73–83 (2009).
Garcia-Castro, M.I., Marcelle, C. & Bronner-Fraser, M. Ectodermal Wnt function as a neural crest inducer. Science 297, 848–851 (2002).
Jiang, X. et al. Isolation and characterization of neural crest stem cells derived from in vitro–differentiated human embryonic stem cells. Stem Cells Dev. 18, 1059–1070 (2009).
Pomp, O. et al. PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Res. 1230, 50–60 (2008).
Heng, H.H., Windle, B. & Tsui, L.C. High-resolution FISH analysis. Curr. Protoc. Hum. Genet. 4.5.1–4.5.23 (2005).
Wesselschmidt, R.L. The teratoma assay: an in vivo assessment of pluripotency. Methods Mol. Biol. 767, 231–241 (2011).
Woo, K. & Fraser, S.E. Order and coherence in the fate map of the zebrafish nervous system. Development 121, 2595–2609 (1995).
Deschene, E.R. & Barresi, M.J. Tissue targeted embryonic chimeras: zebrafish gastrula cell transplantation. J. Vis. Exp. doi:10.3791/1422 (11 September 2009).
Westerfield, M. The Zebrafish Book: A Guide For the Laboratory Use of Zebrafish (Danio rerio) (University of Oregon Press, 2000).
Acknowledgements
This work was supported by a grant to S.D. from the National Institute for General Medical Sciences (GM085354) and a grant to J.D.L. from the Children's Glaucoma Foundation.
Author information
Authors and Affiliations
Contributions
L.M. performed all differentiation experiments, analyzed the data and contributed to writing the manuscript; M.J.K. generated hiPSCs from fibroblasts and performed quality control analysis; S.S.P. maintained patient fibroblasts; A.T.P. designed and performed zebrafish in vivo experiments; J.D.L. designed zebrafish in vivo experiments; M.L.C. supervised the isolation of patient fibroblasts and obtained patient consent; S.D. provided overall direction for the project, analysis of data and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Menendez, L., Kulik, M., Page, A. et al. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc 8, 203–212 (2013). https://doi.org/10.1038/nprot.2012.156
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.156
This article is cited by
-
Biomimetic Strategies for Peripheral Nerve Injury Repair: An Exploration of Microarchitecture and Cellularization
Biomedical Materials & Devices (2023)
-
In vitro modelling of human proprioceptive sensory neurons in the neuromuscular system
Scientific Reports (2022)
-
Human organoids in basic research and clinical applications
Signal Transduction and Targeted Therapy (2022)
-
Glycan Epitope and Integrin Expression Dynamics Characterize Neural Crest Epithelial-to-Mesenchymal Transition (EMT) in Human Pluripotent Stem Cell Differentiation
Stem Cell Reviews and Reports (2022)
-
MAPRE2 mutations result in altered human cranial neural crest migration, underlying craniofacial malformations in CSC-KT syndrome
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.