Abstract
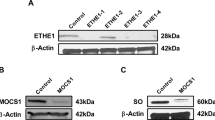
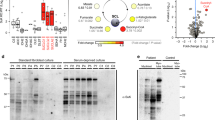
Ethylmalonic encephalopathy is an autosomal recessive, invariably fatal disorder characterized by early-onset encephalopathy, microangiopathy, chronic diarrhea, defective cytochrome c oxidase (COX) in muscle and brain, high concentrations of C4 and C5 acylcarnitines in blood and high excretion of ethylmalonic acid in urine. ETHE1, a gene encoding a β-lactamase–like, iron-coordinating metalloprotein, is mutated in ethylmalonic encephalopathy. In bacteria, ETHE1-like sequences are in the same operon of, or fused with, orthologs of TST, the gene encoding rhodanese, a sulfurtransferase. In eukaryotes, both ETHE1 and rhodanese are located within the mitochondrial matrix. We created a Ethe1−/− mouse that showed the cardinal features of ethylmalonic encephalopathy. We found that thiosulfate was excreted in massive amounts in urine of both Ethe1−/− mice and humans with ethylmalonic encephalopathy. High thiosulfate and sulfide concentrations were present in Ethe1−/− mouse tissues. Sulfide is a powerful inhibitor of COX and short-chain fatty acid oxidation, with vasoactive and vasotoxic effects that explain the microangiopathy in ethylmalonic encephalopathy patients. Sulfide is detoxified by a mitochondrial pathway that includes a sulfur dioxygenase. Sulfur dioxygenase activity was absent in Ethe1−/− mice, whereas it was markedly increased by ETHE1 overexpression in HeLa cells and Escherichia coli. Therefore, ETHE1 is a mitochondrial sulfur dioxygenase involved in catabolism of sulfide that accumulates to toxic levels in ethylmalonic encephalopathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
20 January 2009
In the version of this article initially published online, ‘SDO’ should have been ‘SDH’ in Figure 4a,b. The error has been corrected for the print, PDF and HTML versions of this article.
References
Burlina, A. et al. New clinical phenotype of branched-chain acyl-CoA oxidation defect. Lancet 338, 1522–1523 (1991).
Garcia-Silva, M.T. et al. Encephalopathy, petechiae, and acrocyanosis with ethylmalonic aciduria associated with muscle cytochrome c oxidase deficiency. J. Pediatr. 125, 843–844 (1994).
Gregersen, N. et al. Identification of four new mutations in the short-chain acyl-CoA dehydrogenase (SCAD) gene in two patients: one of the variant alleles, 511C → T, is present at an unexpectedly high frequency in the general population, as was the case for 625G → A, together conferring susceptibility to ethylmalonic aciduria. Hum. Mol. Genet. 7, 619–627 (1998).
Koeberl, D.D. et al. Rare disorders of metabolism with elevated butyryl- and isobutyryl-carnitine detected by tandem mass spectrometry newborn screening. Pediatr. Res. 54, 219–223 (2003).
Sass, J.O. et al. 2-Methylbutyryl-coenzyme A dehydrogenase deficiency: functional and molecular studies on a defect in isoleucine catabolism. Mol. Genet. Metab. 93, 30–35 (2008).
Vockley, J. & Ensenauer, R. Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity. Am. J. Med. Genet. C. Semin. Med. Genet. 142C, 95–103 (2006).
Tiranti, V. et al. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am. J. Hum. Genet. 74, 239–252 (2004).
Tiranti, V. et al. ETHE1 mutations are specific to ethylmalonic encephalopathy. J. Med. Genet. 43, 340–346 (2006).
Mineri, R. et al. Identification of new mutations in the ETHE1 gene in a cohort of 14 patients presenting with ethylmalonic encephalopathy. J. Med. Genet. 45, 473–478 (2008).
Daiyasu, H., Osaka, K., Ishino, Y. & Toh, H. Expansion of the zinc metallo-hydrolase family of the β-lactamase fold. FEBS Lett. 503, 1–6 (2001).
Higashitsuji, H. et al. A novel protein overexpressed in hepatoma accelerates export of NF-κ B from the nucleus and inhibits p53-dependent apoptosis. Cancer Cell 2, 335–346 (2002).
Higashitsuji, H. et al. Enhanced deacetylation of p53 by the anti-apoptotic protein HSCO in association with histone deacetylase 1. J. Biol. Chem. 282, 13716–13725 (2007).
Bensaad, K. & Vousden, K.H. p53: new roles in metabolism. Trends Cell Biol. 17, 286–291 (2007).
Psarra, A.M. & Sekeris, C.E. Nuclear receptors and other nuclear transcription factors in mitochondria: regulatory molecules in a new environment. Biochim. Biophys. Acta 1783, 1–11 (2008).
McCoy, J.G. et al. Structure of an ETHE1-like protein from Arabidopsis thaliana. Acta Crystallogr. D Biol. Crystallogr. 62, 964–970 (2006).
Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 6, 917–935 (2007).
Leschelle, X. et al. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim. Biophys. Acta 1725, 201–212 (2005).
Dorman, D.C. et al. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol. Sci. 65, 18–25 (2002).
Garcia-Silva, M.T., Ribes, A., Campos, Y., Garavaglia, B. & Arenas, J.A. Syndrome of Encephalopathy, Petechiae, and Ethylmalonic Aciduria. Pediatric Neurology. 17, 165–170 (1997).
Furne, J., Springfield, J., Koenig, T., DeMaster, E. & Levitt, M.D. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem. Pharmacol. 62, 255–259 (2001).
Roediger, W.E., Moore, J. & Babidge, W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig. Dis. Sci. 42, 1571–1579 (1997).
Hildebrandt, T.M. & Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 275, 3352–3361 (2008).
Goubern, M., Andriamihaja, M., Nubel, T., Blachier, F. & Bouillaud, F. Sulfide, the first inorganic substrate for human cells. FASEB J. 21, 1699–1706 (2007).
Theissen, U. & Martin, W. Sulfide:quinone oxidoreductase (SQR) from the lugworm Arenicola marina shows cyanide- and thioredoxin-dependent activity. FEBS J. 275, 1131–1139 (2008).
Bella, D.L. & Stipanuk, M.H. Effects of protein, methionine, or chloride on acid-base balance and on cysteine catabolism. Am. J. Physiol. 269, E910–E917 (1995).
Fernandez-Silva, P., Martinez-Azorin, F., Micol, V. & Attardi, G. The human mitochondrial transcription termination factor (mTERF) is a multizipper protein but binds to DNA as a monomer, with evidence pointing to intramolecular leucine zipper interactions. EMBO J. 16, 1066–1079 (1997).
Tiranti, V. et al. Identification of the gene encoding the human mitochondrial RNA polymerase (h-mtRPOL) by cyberscreening of the Expressed Sequence Tags database. Hum. Mol. Genet. 6, 615–625 (1997).
Moroni, I., D'Incerti, L., Farina, L., Rimoldi, M. & Uziel, G. Clinical, biochemical and neuroradiological findings in l-2-hydroxyglutaric aciduria. Neurol. Sci. 21, 103–108 (2000).
Di Donato, S. et al. Systemic carnitine deficiency due to lack of electron transfer flavoprotein:ubiquinone oxidoreductase. Neurology 36, 957–963 (1986).
Shih, V.E., Carney, M.M. & Mandell, R. A simple screening test for sulfite oxidase deficiency: detection of urinary thiosulfate by a modification of Sorbo's method. Clin. Chim. Acta 95, 143–145 (1979).
Linden, D.R. et al. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J. Neurochem. 106, 1577–1585 (2008).
Furne, J., Saeed, A. & Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1479–R1485 (2008).
Bennett, M.J. et al. Glutaric aciduria type II: biochemical investigation and treatment of a child diagnosed prenatally. J. Inherit. Metab. Dis. 7, 57–61 (1984).
Sciacco, M. & Bonilla, E. Cytochemistry and immunocytochemistry of mitochondria in tissue sections. Methods Enzymol. 264, 509–521 (1996).
Bugiani, M. et al. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta 1659, 136–147 (2004).
Sottocasa, G.L., Kuylenstierna, B., Ernster, L. & Bergstrand, A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J. Cell Biol. 32, 415–438 (1967).
Sörbo, B. Sulfate: turbidimetric and nephelometric methods. Methods Enzymol. 143, 3–6 (1987).
Sörbo, B. Rhodanese. Methods Enzymol. 2, 334–337 (1955).
Dell'Agnello, C. et al. Increased longevity and refractoriness to Ca2+-dependent neurodegeneration in Surf1 knockout mice. Hum. Mol. Genet. 16, 431–444 (2007).
Acknowledgements
We thank A. Bradley (The Wellcome Trust Sanger Institute) for AB1 mouse embryonic stem cells. We are grateful to M. Bada for skillful technical assistance; to B. Garavaglia, E. Lamantea, F. Forlani and M.K. Grieshaber for valuable discussion; to the Chemical Analysis Laboratory, University of Georgia, for metal analysis; and to Primm for MALDI TOF mass spectometry analysis. This work was supported by the Pierfranco and Luisa Mariani Foundation Italy, Fondazione Telethon-Italy grant number GGP07019, the Italian Ministry of University and Research (FIRB 2003—project RBLA038RMA), MITOCIRCLE and EUMITOCOMBAT network grants from the European Union framework program 6 and by the Deutsche Forschungsgemeinschaft (GR 456/22-1).
Author information
Authors and Affiliations
Contributions
V.T. and M.Z. designed the experimental plan and wrote the manuscript; C.V. and C.T. took care of the creation of the Ethe1-knockout mouse; C.V. characterized the mouse phenotype; I.DM. carried out the measurements of the mitochondrial respiratory chain biochemistry; R.M. performed the experiments on expression and purification of the recombinant ETHE1 protein in E. coli; T.H. performed the sulfur-related enzymology; M.D.L. measured the sulfur compounds in body fluids and tissues; G.F. and A.P. carried out the morphological investigation; and M.R. took care of the metabolite analysis in urine and blood.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figs. 1–5 (PDF 2608 kb)
Rights and permissions
About this article
Cite this article
Tiranti, V., Viscomi, C., Hildebrandt, T. et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med 15, 200–205 (2009). https://doi.org/10.1038/nm.1907
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1907
This article is cited by
-
Sulfide regulation of cardiovascular function in health and disease
Nature Reviews Cardiology (2023)
-
Ethylmalonic Encephalopathy: a literature review and two new cases of mild phenotype
Neurological Sciences (2023)
-
AAV-vector based gene therapy for mitochondrial disease: progress and future perspectives
Orphanet Journal of Rare Diseases (2022)
-
Mitochondrial Dysfunction and Redox Homeostasis Impairment as Pathomechanisms of Brain Damage in Ethylmalonic Encephalopathy: Insights from Animal and Human Studies
Cellular and Molecular Neurobiology (2022)
-
Defense responses of sulfur dioxygenase to sulfide stress in the razor clam Sinonovacula constricta
Genes & Genomics (2021)