Abstract
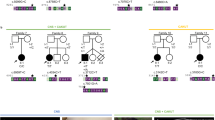
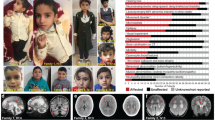
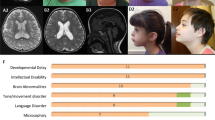
Meckel-Gruber syndrome is a severe autosomal, recessively inherited disorder characterized by bilateral renal cystic dysplasia, developmental defects of the central nervous system (most commonly occipital encephalocele), hepatic ductal dysplasia and cysts and polydactyly1,2,3. MKS is genetically heterogeneous, with three loci mapped: MKS1, 17q21-24 (ref. 4); MKS2, 11q13 (ref. 5) and MKS3 (ref. 6). We have refined MKS3 mapping to a 12.67-Mb interval (8q21.13-q22.1) that is syntenic to the Wpk locus in rat, which is a model with polycystic kidney disease, agenesis of the corpus callosum and hydrocephalus7,8. Positional cloning of the Wpk gene suggested a MKS3 candidate gene, TMEM67, for which we identified pathogenic mutations for five MKS3-linked consanguineous families. MKS3 is a previously uncharacterized, evolutionarily conserved gene that is expressed at moderate levels in fetal brain, liver and kidney but has widespread, low levels of expression. It encodes a 995–amino acid seven-transmembrane receptor protein of unknown function that we have called meckelin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
GenBank/EMBL/DDBJ
References
Ahdab-Barmada, M. & Claassen, D. A distinctive triad of malformations of the central nervous system in the Meckel-Gruber syndrome. J. Neuropathol. Exp. Neurol. 49, 610–620 (1990).
Salonen, R. & Paavola, P. Meckel syndrome. J. Med. Genet. 35, 497–501 (1998).
Al-Gazali, L.I., Abdel Raziq, A., Al-Shather, W., Shahzadi, R. & Azhar, N. Meckel syndrome and Dandy Walker malformation. Clin. Dysmorphol. 5, 73–76 (1996).
Paavola, P., Salonen, R., Weissenbach, J. & Peltonen, L. The locus for Meckel syndrome with multiple congenital anomalies maps to chromosome 17q21-q24. Nat. Genet. 11, 213–215 (1995).
Roume, J. et al. A gene for Meckel syndrome maps to chromosome 11q13. Am. J. Hum. Genet. 63, 1095–1101 (1998).
Morgan, N.V. et al. A novel locus for Meckel-Gruber syndrome, MKS3, maps to chromosome 8q24. Hum. Genet. 111, 456–461 (2002).
Nauta, J. et al. New rat model that phenotypically resembles autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 11, 2272–2284 (2000).
Gattone, V.H. II et al. Development of multiorgan pathology in the wpk rat model of polycystic kidney disease. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 277, 384–395 (2004).
Fraser, F.C. & Lytwyn, A. Spectrum of anomalies in the Meckel syndrome or: “Maybe there is a malformation syndrome with at least one constant anomaly. Am. J. Med. Genet. 9, 67–73 (1981).
Salonen, R. The Meckel syndrome: clinicopathological findings in 67 patients. Am. J. Med. Genet. 18, 671–689 (1984).
Blankenberg, T.A., Ruebner, B.H., Ellis, W.G., Bernstein, J. & Dimmick, J.E. Pathology of renal and hepatic anomalies in Meckel syndrome. Am. J. Med. Genet. 3 (Suppl.), 395–410 (1987).
Simpson, J.L. et al. Genetic heterogeneity in neural tube defects. Ann. Genet. 34, 279–286 (1991).
Xu, Y.K. & Nusse, R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr. Biol. 8, R405–R406 (1998).
Cadigan, K.M. & Nusse, R. Wnt signaling: a common theme in animal development. Genes Dev. 11, 3286–3305 (1997).
Jenny, A., Reynolds-Kenneally, J., Das, G., Burnett, M. & Mlodzik, M. Diego and Prickle regulate Frizzled planar cell polarity signaling by competing for Dishevelled binding. Nat. Cell Biol. 7, 691–697 (2005).
Povelones, M., Howes, R., Fish, M. & Nusse, R. Genetic evidence that Drosophila Frizzled controls planar cell polarity and Armadillo signaling by a common mechanism. Genetics (in the press).
Ross, A.J. et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135–1140 (2005).
Ansley, S.J. et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425, 628–633 (2003).
Beales, P.L. Lifting the lid on Pandora's box: the Bardet-Biedl syndrome. Curr. Opin. Genet. Dev. 15, 315–323 (2005).
Karmous-Benailly, H. et al. Antenatal presentation of Bardet-Biedl syndrome may mimic Meckel syndrome. Am. J. Hum. Genet. 76, 493–504 (2005).
Badano, J.L., Teslovich, T.M. & Katsanis, N. The centrosome in human genetic disease. Nat. Rev. Genet. 6, 194–205 (2005).
Calvet, J.P. New insights into ciliary function: kidney cysts and photoreceptors. Proc. Natl. Acad. Sci. USA 100, 5583–5585 (2003).
Pazour, G.J. Comparative genomics: prediction of the ciliary and basal body proteome. Curr. Biol. 14, R575–R577 (2004).
Efimenko, E. et al. Analysis of xbx genes in C. elegans. Development 132, 1923–1934 (2005).
Sambrook, J. & Russell, D.W. Molecular Cloning, a Laboratory Manual 3rd edn. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2001).
Consugar, M.B. et al. Haplotype analysis improves molecular diagnostics of autosomal recessive polycystic kidney disease. Am. J. Kidney Dis. 45, 77–87 (2005).
Maina, E.N. et al. Identification of novel VHL target genes and relationship to hypoxic response pathways. Oncogene 24, 4549–4558 (2005).
Bateman, A. et al. The Pfam protein families database. Nucleic Acids Res. 28, 263–266 (2000).
Letunic, I. et al. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32, D142–D144 (2004).
Blom, N., Gammeltoft, S. & Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362 (1999).
Acknowledgements
We thank the MKS families for their generous help. We are grateful to M. Barr for useful discussions. This research was supported by grants from the Wellcome Trust to R.C.T. and E.R.M.; by grants to C.A.J. from the UK Birth Defects Foundation, University of Birmingham Medical School Scientific Projects and Birmingham Women's Hospital Research Fund; and by grants from the US National Institutes of Health, the PKD Foundation and the Mayo Foundation to V.H.G. and to P.C.H.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Sequence alignment of meckelin proteins from human, rat, mouse, chicken and Tetraodon nigroviridis. (PDF 1360 kb)
Supplementary Table 1
Intron/exon structure of MKS3/TMEM67 and Mks3/Tmem67. (PDF 99 kb)
Supplementary Table 2
Novel human chromosome 8 microsatellite markers for the MKS3 locus and novel rat chromosme 5 microsatellite markers for the Wpk locus. (PDF 86 kb)
Supplementary Table 3
Sequencing primers for the MKS3/TMEM67 gene. (PDF 75 kb)
Rights and permissions
About this article
Cite this article
Smith, U., Consugar, M., Tee, L. et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet 38, 191–196 (2006). https://doi.org/10.1038/ng1713
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1713
This article is cited by
-
Neurosurgical intervention for the Meckel-Gruber Syndrome: A systematic review
Child's Nervous System (2024)
-
Inhibition of serum- and glucocorticoid-induced kinase 1 ameliorates hydrocephalus in preclinical models
Fluids and Barriers of the CNS (2023)
-
A loss of function variant in AGPAT3 underlies intellectual disability and retinitis pigmentosa (IDRP) syndrome
European Journal of Human Genetics (2023)
-
Nephronophthisis: a pathological and genetic perspective
Pediatric Nephrology (2023)
-
LPA signaling acts as a cell-extrinsic mechanism to initiate cilia disassembly and promote neurogenesis
Nature Communications (2021)