Abstract
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is associated with fibrofatty replacement of cardiac myocytes, ventricular tachyarrhythmias and sudden cardiac death. In 32 of 120 unrelated individuals with ARVC, we identified heterozygous mutations in PKP2, which encodes plakophilin-2, an essential armadillo-repeat protein of the cardiac desmosome. In two kindreds with ARVC, disease was incompletely penetrant in most carriers of PKP2 mutations.
Similar content being viewed by others
Main
Individuals with ARVC may present with palpitations or ventricular tachyarrhythmias that originate from the right ventricle and may lead to syncope or sudden cardiac death, a relatively common and the most feared clinical manifestation of the disease1,2,3. Localized or diffuse atrophy and progressive replacement of right ventricular myocytes with fatty or fibrofatty tissue is the pathological hallmark of ARVC. Although right ventricular involvement predominates, the left ventricle also can be affected. Heart failure of the right and, less commonly, the left ventricle may occur later in the disease process. The exact prevalence and incidence of ARVC is unknown. Familial disease accounts for an unknown proportion of ARVC, with estimates ranging from 30% to 80%. Eight genomic loci and rare mutations in plakoglobin (JUP), desmoplakin (DSP) and the cardiac ryanodine receptor (RYR2) have been reported in nonsyndromic and syndromic cases2.
Desmosomes are complex multiprotein structures of the cell membrane and provide structural and functional integrity to adjacent cells (e.g., epithelial cells and cardiomyocytes4). Desmosomal proteins also have a role in cell signaling5,6. At least three groups of molecules contribute to the formation of desmosomes: desmosomal cadherins, armadillo-repeat proteins and plakins7. The plakophilins, which are armadillo-related proteins, contain ten 42–amino acid armadillo-repeat motifs and are located in the outer dense plaque of desmosomes linking desmosomal cadherins with desmoplakin and the intermediate filament system8. Like other armadillo-repeat proteins, plakophilins are also found in the nucleus, where they may have a role in transcriptional regulation9. Plakophilin-2 exists in two alternatively spliced isoforms (2a and 2b), interacts with multiple other cell adhesion proteins and is the primary cardiac plakophilin8,10.
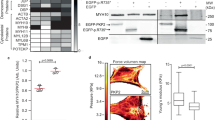
On the basis of findings of a lethal defect in cardiac morphogenesis at embryonic day 10.75 in mice homozygous with respect to a deletion mutation of Pkp2 (ref. 11), we hypothesized that mutations in human PKP2 may account for ARVC. A total of 120 unrelated probands of western European descent (101 males and 19 females) were admitted to tertiary referral centers and diagnosed with ARVC in accordance with clinical criteria proposed by a Task Force12. We directly sequenced all 14 PKP2 exons, including flanking intronic splice sequences (Supplementary Methods online), and identified 25 different heterozygous mutations in 32 probands (27 males and 5 females). Of the 25 PKP2 mutations, 12 were insertion-deletion mutations, 6 were nonsense mutations, 4 were missense mutations and 3 were splice site mutations (Table 1 and Supplementary Figs. 1 and 2 online). Although most mutations were detected in the C-terminal half of the molecule, mutations were located throughout the gene. We observed one PKP2 splice acceptor site mutation (2146−1G → C) and two nonsense mutations (235C → T and 2203C → T) in several unrelated individuals with ARVC (Table 1). Genetic analysis of microsatellite DNA markers at or near the PKP2 locus on chromosome 12p11 did not identify shared haplotypes between the ARVC cases with identical mutations (data not shown). Because parental genotypes were not available, however, we could not carry out formal segregation analyses. Therefore, ancient founder mutations cannot be excluded with certainty. Six instances of a nonsense mutation in PKP2 (235C → T) indicated that nucleotide residue 235 might be a mutational hot spot. None of the 25 PKP2 mutations were observed in 500 control chromosomes.
Although familial disease was not systematically evaluated in all probands, members of two kindreds (kindreds A100 and EPF; Fig. 1) were available for a detailed clinical and molecular analysis. A 2-bp deletion in PKP2 exon 10 (2076_2077delAA) is predicted to cause the addition of 48 amino acid residues before a premature stop signal is introduced (C693fsX741); we observed this mutation in two clinically affected members of kindred A100 (individuals 18 and 161; Fig. 1 and Supplementary Table 1 online). We carried out western-blot analysis of a right ventricular biopsy specimen obtained from individual A100/161 but did not detect the truncated plakophilin-2 protein (expected size of truncated PKP2, 82.1 kDa; normal PKP2, 97.4 kDa). The specimen did contain less wild-type plakophilin-2 than control myocardium (Supplementary Fig. 3 online), implicating haploinsufficiency as the operant mechanism in this PKP2 mutation.
Black, white, hatched and gray symbols represent clinically affected individuals, unaffected individuals, individuals of undetermined disease status and individuals of unknown disease status, respectively. Presence (+) or absence (−) of the PKP2 mutation in those individuals available for mutation analysis is indicated. N/A, not available for genetic diagnosis. Arrows indicate index individuals. Diagnostic scores refer to proposed clinical criteria12.
Clinical evaluation of first-degree relatives of kindred A100 identified two individuals (individuals 18 and 161; Fig. 1 and Supplementary Table 1 online) who are affected by ARVC in accordance with the Task Force criteria12. Although four other members of kindred A100 also carried the 2-bp deletion mutation in PKP2, they have normal or mild disease phenotypes, even at more advanced ages (e.g., individual 1). Two members of another family with ARVC (EPF) died suddenly at ages 16 years and 15 years (individuals 11 and 12; Fig. 1). An autopsy study showed that both individuals had ARVC. Of the five surviving mutation carriers in kindred EPF, only one showed clinical manifestations diagnostic of ARVC (individual EPF/17; Supplementary Table 1 online). The reason for this incomplete penetrance in kindreds A100 and EPF is unknown, but it may be related to gender, genetic or epigenetic modifiers or other unknown factors (e.g., viral infection as trigger for disease). Although transmission of disease in kindreds A100 and EPF is compatible with autosomal dominance, linkage analysis would have been difficult due to incomplete penetrance of disease in both kindreds (<0.5). ARVC in kindred EPF has been previously reported to be linked to chromosome 2q32.1–q32.3 (ref. 13). The identification of mutation 216insG in PKP2 in this kindred, coupled with the absence in any individual family of statistical significance for the original linkage to chromosome 2, implies that assignment of ARVC to chromosome 2 on the basis of aggregation of lod scores from three small families should possibly be considered erroneous.
Plakophilins, together with the other desmosomal proteins, assemble to form cell adhesion complexes that carry out diverse structural and functional tasks. These include mechanically safeguarding cellular and organ architecture by transmitting force between cells and participating in several signal transduction pathways. Support for the idea that plakophilins have an essential role in desmosome formation and function comes from this and two other reports: (i) the identification of recessive mutations in PKP1 that cause ectodermal dysplasia/skin fragility syndrome, a rare skin disorder characterized by small, poorly formed desmosomes and perturbed desmosome-keratin intermediate filament interactions14; and (ii) the ablation of mouse Pkp2, the main plakophilin in cardiac muscle, which results in defects of heart morphogenesis and junctional architecture with embryonic lethality at midgestation11. Therefore, plakophilin-1 and plakophilin-2 have essential roles in desmosome formation and in skin and heart development, respectively.
How PKP2 mutations perturb cardiac desmosome assembly and function in ARVC is unknown. We speculate that lack of plakophilin-2 or incorporation of mutant plakophilin-2 into cardiac desmosomes impairs cell-cell contacts and, as a consequence, disrupts adjacent cardiomyocytes, particularly in response to mechanical stress or stretch (thus providing a potential explanation for the high prevalence of the disorder in athletes, the frequent occurrence of ventricular tachyarrhythmias and sudden death during exercise and the predominant affection of the right ventricle). Intercellular disruption would occur first in areas of high stress and stretch: the right ventricular outflow tract, apex and inferobasal (subtricuspid) area, which are pathological predilection areas in ARVC (forming the 'triangle of dysplasia'15). The potential cellular mechanism for the initiation of ventricular tachyarrhythmias in ARVC is the intrinsic variation in conduction properties as a result of these patchy areas of fibrofatty myocyte degeneration.
Because mutations causing ARVC have been identified in PKP2 (plakophilin-2), JUP (plakoglobin) and DSP (desmoplakin), ARVC may be considered as a disease of the desmosome.
All genetic analyses were done after informed consent was obtained from the participating individuals. The local ethical committees from Humboldt University Berlin, University of Münster, Weill Medical College of Cornell University and Harvard University approved the experimental plan.
GenBank accession number. PKP2, X97675.
Note: Supplementary information is available on the Nature Genetics website.
Accession codes
References
Thiene, G., Nava, A., Corrado, D., Rossi, L. & Pennelli, N. N. Engl. J. Med. 318, 129–133 (1988).
Paul, M., Schulze-Bahr, E., Breithardt, G. & Wichter, T.A. Z. Kardiol. 92, 128–136 (2003).
Fontaine, G. et al. Annu. Rev. Med. 50, 17–35 (1999).
Green, K.J. & Gaudry, C.A. Nat. Rev. Mol. Cell Biol. 1, 208–216 (2000).
Jamora, C. & Fuchs, E. Nat. Cell Biol. 4, E101–E108 (2002).
Ko, K.S., Arora, P.D. & McCulloch, C.A. J. Biol. Chem. 276, 35967–35977 (2001).
Garrod, D.R., Merritt, A.J. & Nie, Z. Curr. Opin. Cell Biol. 14, 537–545 (2002).
Mertens, C., Kuhn, C. & Franke, W.W. J. Cell Biol. 135, 1009–1025 (1996).
Mertens, C. et al. Proc. Natl. Acad. Sci. USA 98, 7795–7800 (2001).
Chen, X., Bonne, S., Hatzfeld, M., van Roy, F. & Green, K.J. J. Biol. Chem. 277, 10512–10522 (2002).
Grossmann, K. et al. J. Cell Biol. (in the press).
McKenna, W.J. et al. Br. Heart J. 71, 215–218 (1994).
Rampazzo, A. et al. Genomics 45, 259–263 (1997).
McGrath, J.A. et al. Nat. Genet. 17, 240–244 (1997).
Marcus, F.I. et al. Circulation 65, 384–398 (1982).
Acknowledgements
We thank I. Trippmacher, S. Milan, E. Schulze-Bahr, P. Gerdes, S. Pereira and S. Helms for technical help; J. Waigand for carrying out the cardiac biopsies; and J. Schulz-Menger, B. Pilz, B. Keweloh, B. Struk, A. Schirdewan and H. Peters for logistic help. This work was supported by grants from the Ernst und Berta Grimmke Stiftung, Düsseldorf, Germany (L.T.); the Deutsche Forschungsgemeinschaft, Bonn, Germany (T.W.); the Fondation Leducq, Paris, France (E.S.-B.); the European Commission, Brussels, Belgium (T.W.); and the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland, USA (C.T.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
PKP2 structure and mutations associated with ARVC. (PDF 180 kb)
Supplementary Fig. 2
PKP2 splice site mutations. (PDF 1016 kb)
Supplementary Fig. 3
Cardiac western blot analysis of PKP2 mutation 2076_2077delAA. (PDF 140 kb)
Supplementary Table 1
Characteristics of clinically, anatomically, and/or genetically affected individuals of ARVC kindreds A100 and EPF. (PDF 2 kb)
Rights and permissions
About this article
Cite this article
Gerull, B., Heuser, A., Wichter, T. et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 36, 1162–1164 (2004). https://doi.org/10.1038/ng1461
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1461
This article is cited by
-
A truncating variant altering the extreme C-terminal region of desmoplakin (DSP) suggests the crucial functional role of the region: a case report study
BMC Medical Genomics (2023)
-
Genomic approaches to identify and investigate genes associated with atrial fibrillation and heart failure susceptibility
Human Genomics (2023)
-
Restoring PKP2 in arrhythmogenic cardiomyopathy
Nature Cardiovascular Research (2023)
-
Plakophilin 2 gene therapy prevents and rescues arrhythmogenic right ventricular cardiomyopathy in a mouse model harboring patient genetics
Nature Cardiovascular Research (2023)
-
A novel DSP zebrafish model reveals training- and drug-induced modulation of arrhythmogenic cardiomyopathy phenotypes
Cell Death Discovery (2023)