Abstract
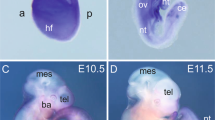
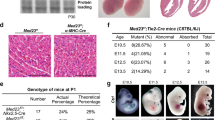
Neurofibromatosis type 1 (NF1) or von Recklinghausen neurofibromatosis is a genetic disorder that occurs in 1 of 4000 births and is characterized by benign and malignant tumors. Cardiovascular defects also contribute to NF1, though the pathogenesis is still unclear. Deficiency in neurofibromin (encoded by Nf1) in mice results in mid-embryonic lethality owing to cardiac abnormalities previously thought to be secondary to cardiac neural-crest defects. Using tissue-specific gene inactivation, we show that endothelial-specific inactivation of Nf1 recapitulates key aspects of the complete null phenotype, including multiple cardiovascular abnormalities involving the endocardial cushions and myocardium. This phenotype is associated with an elevated level of ras signaling in Nf1−/− endothelial cells and greater nuclear localization of the transcription factor Nfatc1. Inactivation of Nf1 in the neural crest does not cause cardiac defects but results in tumors of neural-crest origin resembling those seen in humans with NF1. These results establish a new and essential role for Nf1 in endothelial cells and confirm the requirement for neurofibromin in the neural crest.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friedman, J.M. et al. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet. Med. 4, 105–111 (2002).
Lin, A.E. et al. Cardiovascular malformations and other cardiovascular abnormalities in neurofibromatosis 1. Am. J. Med. Genet. 95, 108–117 (2000).
Jacks, T. et al. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat. Genet. 7, 353–361 (1994).
Brannan, C.I. et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 8, 1019–1029 (1994).
Lakkis, M.M. & Epstein, J.A. Neurofibromin modulation of ras activity is required for normal endocardial–mesenchymal transformation in the developing heart. Development 125, 4359–4367 (1998).
Kirby, M.L., Gale, T.F. & Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 220, 1059–1061 (1983).
Zhu, Y. et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 15, 859–876 (2001).
Kisanuki, Y.Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230–242 (2001).
Meyer, D. & Birchmeier, C. Multiple essential functions of neuregulin in development. Nature 378, 386–390 (1995).
Erickson, S.L. et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development 124, 4999–5011 (1997).
Morrison-Graham, K., Schatteman, G., Bork, T., Bowen-Pope, D. & Weston, J. A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest–derived cells. Development 115, 133–142 (1992).
Epstein, J. Pax3, neural crest and cardiovascular development. Trends Cardiovasc. Med. 6, 255–261 (1996).
Feiner, L. et al. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128, 3061–3070 (2001).
Galvin, K.M. et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 24, 171–174 (2000).
Ya, J. et al. Sox4-deficiency syndrome in mice is an animal model for common trunk. Circ. Res. 83, 986–994 (1998).
Yang, J.T., Rayburn, H. & Hynes, R.O. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 121, 549–560 (1995).
de la Pompa, J.L. et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392, 182–186 (1998).
Camenisch, T.D. et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106, 349–360 (2000).
Chen, B. et al. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 24, 296–299 (2000).
Ranger, A.M. et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 392, 186–190 (1998).
Ichida, M. & Finkel, T. Ras regulates NFAT3 activity in cardiac myocytes. J. Biol. Chem. 276, 3524–3530 (2001).
Woodrow, M.A., Rayter, S., Downward, J. & Cantrell, D.A. p21ras function is important for T-cell antigen receptor and protein kinase C regulation of nuclear factor of activated T cells. J. Immunol. 150, 3853–3861 (1993).
Vogel, K.S. et al. Mouse tumor model for neurofibromatosis type 1. Science 286, 2176–2179 (1999).
Cichowski, K. et al. Mouse models of tumor development in neurofibromatosis type 1. Science 286, 2172–2176 (1999).
Jiang, X., Rowitch, D.H., Soriano, P., McMahon, A.P. & Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 127, 1607–1616 (2000).
Li, J., Chen, F. & Epstein, J.A. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis 26, 162–164 (2000).
Yamauchi, Y. et al. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev. Biol. 212, 191–203 (1999).
Ricci, A. Jr. et al. Malignant peripheral nerve sheath tumors arising from ganglioneuromas. Am. J. Surg. Pathol. 8, 19–29 (1984).
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Acknowledgements
We are grateful to M. Yanagisawa for providing Tek–cre mice; B. Zhou for providing the Nfatc1 expression plasmid; L. Rorke for reviewing pathologic specimens; and M. Weiss, C. Thompson, C. Simon, E. Morrisey, M. Kahn and members of J.A.E.'s laboratory for critically reading the manuscript and for helpful discussions. This work was supported by grants from the WW Smith Foundation, the American Heart Association and the US National Institutes of Health to J.A.E. L.F.P. is supported by grants from the US National Institute of Neurological Disorders and Stroke and the US Department of Defense. A.D.G. is supported by the Department of Cell and Developmental Biology predoctoral training grant from the US National Institutes of Health. Y.Z. is a recipient of a Young Investigator Award from the National Neurofibromatosis Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gitler, A., Zhu, Y., Ismat, F. et al. Nf1 has an essential role in endothelial cells. Nat Genet 33, 75–79 (2003). https://doi.org/10.1038/ng1059
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1059
This article is cited by
-
An Assessment of the Therapeutic Landscape for the Treatment of Heart Disease in the RASopathies
Cardiovascular Drugs and Therapy (2023)
-
Functional and morphological cardiovascular alterations associated with neurofibromatosis 1
Scientific Reports (2020)
-
Increased extracellular matrix deposition during chondrogenic differentiation of dental pulp stem cells from individuals with neurofibromatosis type 1: an in vitro 2D and 3D study
Orphanet Journal of Rare Diseases (2018)
-
Cellular interplay via cytokine hierarchy causes pathological cardiac hypertrophy in RAF1-mutant Noonan syndrome
Nature Communications (2017)
-
Emerging genotype–phenotype relationships in patients with large NF1 deletions
Human Genetics (2017)