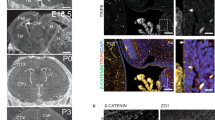
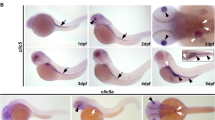
Abstract
Mutations affecting ciliary components cause ciliopathies. As described here, we investigated Tectonic1 (Tctn1), a regulator of mouse Hedgehog signaling, and found that it is essential for ciliogenesis in some, but not all, tissues. Cell types that do not require Tctn1 for ciliogenesis require it to localize select membrane-associated proteins to the cilium, including Arl13b, AC3, Smoothened and Pkd2. Tctn1 forms a complex with multiple ciliopathy proteins associated with Meckel and Joubert syndromes, including Mks1, Tmem216, Tmem67, Cep290, B9d1, Tctn2 and Cc2d2a. Components of this complex co-localize at the transition zone, a region between the basal body and ciliary axoneme. Like Tctn1, loss of Tctn2, Tmem67 or Cc2d2a causes tissue-specific defects in ciliogenesis and ciliary membrane composition. Consistent with a shared function for complex components, we identified a mutation in TCTN1 that causes Joubert syndrome. Thus, a transition zone complex of Meckel and Joubert syndrome proteins regulates ciliary assembly and trafficking, suggesting that transition zone dysfunction is the cause of these ciliopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hodges, M.E., Scheumann, N., Wickstead, B., Langdale, J.A. & Gull, K. Reconstructing the evolutionary history of the centriole from protein components. J. Cell Sci. 123, 1407–1413 (2010).
Goetz, S.C. & Anderson, K.V. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344 (2010).
Sorokin, S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363–377 (1962).
Nachury, M.V., Seeley, E.S. & Jin, H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59–87 (2010).
Sang, L. et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145, 513–528 (2011).
Williams, C.L. et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023–1041 (2011).
Craige, B. et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927–940 (2010).
Hu, Q. et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 (2010).
Tobin, J.L. & Beales, P.L. The nonmotile ciliopathies. Genet. Med. 11, 386–402 (2009).
Hildebrandt, F., Benzing, T. & Katsanis, N. Ciliopathies. N. Engl. J. Med. 364, 1533–1543 (2011).
Shaheen, R. et al. A TCTN2 mutation defines a novel Meckel Gruber syndrome locus. Hum. Mutat. 32, 573–578 (2011).
Leitch, C.C. et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat. Genet. 40, 443–448 (2008).
Khanna, H. et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat. Genet. 41, 739–745 (2009).
Geng, L. et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 119, 1383–1395 (2006).
Mazelova, J. et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 28, 183–192 (2009).
Jin, H. et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141, 1208–1219 (2010).
Nachury, M.V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Berbari, N.F., Lewis, J.S., Bishop, G.A., Askwith, C.C. & Mykytyn, K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA 105, 4242–4246 (2008).
Mykytyn, K. et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc. Natl. Acad. Sci. USA 101, 8664–8669 (2004).
Corbit, K.C. et al. Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005).
Boehlke, C. et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat. Cell Biol. 12, 1115–1122 (2010).
Reiter, J.F. & Skarnes, W.C. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev. 20, 22–27 (2006).
Caspary, T., Larkins, C.E. & Anderson, K.V. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell 12, 767–778 (2007).
Cevik, S. et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J. Cell Biol. 188, 953–969 (2010).
Weatherbee, S.D., Niswander, L.A. & Anderson, K.V. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum. Mol. Genet. 18, 4565–4575 (2009).
Jonassen, J.A., San Agustin, J., Follit, J.A. & Pazour, G.J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 183, 377–384 (2008).
Follit, J.A. et al. Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 4, e1000315 (2008).
Huangfu, D. & Anderson, K.V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325–11330 (2005).
Liu, A., Wang, B. & Niswander, L.A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132, 3103–3111 (2005).
Bénazet, J.D. & Zeller, R. Vertebrate limb development: moving from classical morphogen gradients to an integrated 4-dimensional patterning system. Cold Spring Harb. Perspect. Biol. 1, a001339 (2009).
Torres, J.Z., Miller, J.J. & Jackson, P.K. High-throughput generation of tagged stable cell lines for proteomic analysis. Proteomics 9, 2888–2891 (2009).
Hopp, K. et al. B9D1 is revealed as a novel Meckel syndrome (MKS) gene by targeted exon-enriched next-generation sequencing and deletion analysis. Hum. Mol. Genet. 20, 2524–2534 (2011).
Won, J. et al. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Hum. Mol. Genet. 20, 482–496 (2011).
Holopainen, J.M. et al. Interaction and localization of the retinitis pigmentosa protein RP2 and NSF in retinal photoreceptor cells. Biochemistry 49, 7439–7447 (2010).
Cook, S.A. et al. A mouse model for Meckel syndrome type 3. J. Am. Soc. Nephrol. 20, 753–764 (2009).
Dawe, H.R. et al. The Meckel-Gruber syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum. Mol. Genet. 16, 173–186 (2007).
Bishop, G.A., Berbari, N.F., Lewis, J. & Mykytyn, K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 505, 562–571 (2007).
Pazour, G.J., San Agustin, J.T., Follit, J.A., Rosenbaum, J.L. & Witman, G.B. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in Orpk mice with polycystic kidney disease. Curr. Biol. 12, R378–R380 (2002).
Chen, J.K., Taipale, J., Young, K.E., Maiti, T. & Beachy, P.A. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 99, 14071–14076 (2002).
Hildebrandt, F. et al. A systematic approach to mapping recessive disease genes in individuals from outbred populations. PLoS Genet. 5, e1000353 (2009).
Cantagrel, V. et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 83, 170–179 (2008).
Vierkotten, J., Dildrop, R., Peters, T., Wang, B. & Rüther, U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 134, 2569–2577 (2007).
Lechtreck, K.F. et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187, 1117–1132 (2009).
Geimer, S. & Melkonian, M. The ultrastructure of the Chlamydomonas reinhardtii basal apparatus: identification of an early marker of radial asymmetry inherent in the basal body. J. Cell Sci. 117, 2663–2674 (2004).
Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. Manipulating the Mouse Embryo: a Laboratory Manual 3rd edn. (Cold Spring Harbor Lab Press, Cold Spring Harbor, New York, USA, 2002).
Kruglyak, L. & Daly, M.J. Linkage thresholds for two-stage genome scans. Am. J. Hum. Genet. 62, 994–997 (1998).
Strauch, K. et al. Parametric and nonparametric multipoint linkage analysis with imprinting and two-locus-trait models: application to mite sensitization. Am. J. Hum. Genet. 66, 1945–1957 (2000).
Gudbjartsson, D.F., Jonasson, K., Frigge, M.L. & Kong, A. Allegro, a new computer program for multipoint linkage analysis. Nat. Genet. 25, 12–13 (2000).
Otto, E.A. et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat. Genet. 42, 840–850 (2010).
Acknowledgements
We thank the Institute for Regeneration Medicine and Nikon Imaging Centers at University of California San Francisco. The Servei Central de Suport a la Investigació Experimental and M. Soriano-Navarro assisted with electron microscopy. Members of the Reiter lab provided valuable discussions. K. Anderson, D. Beier, J. Gleeson, C. Johnson, H. Khanna, R. Molday, K. Mykytyn, G. Pazour, S. Scales, J. Sillibourne and S. Somlo provided antibodies or plasmids. T.R.N. is supported by a National Science Foundation predoctoral grant and an National Institute for General Medical Sciences-Initiative for Maximizing Student Diversity grant (R25-GM56847). N.K. is a Distinguished George W. Brumley Professor. F.H. is an investigator of the Howard Hughes Medical Institute, a Doris Duke Distinguished Clinical Scientist and a Frederick G. L. Huetwell Professor. This work was funded by grants from the US National Institutes of Health to N.K. (HD04260, DK072301, DK075972), F.H. (DK1069274, DK1068306, RC4-K090917) and to J.F. Reiter (AR054396), and from the March of Dimes, the Burroughs Wellcome Fund, the Packard Foundation and the Sandler Family Supporting Foundation to J.F. Reiter.
Author information
Authors and Affiliations
Contributions
F.R.G.-G. and K.C.C. performed most of the experiments and wrote the manuscript. M.S.S.-P. did most of the electron microscopy and was supervised by J.M.G.-V. Human genetics were supervised by F.H. and N.K. and performed by G.R., E.A.O., D.J.J., C.L.B. and J.F. Robinson. T.R.N. performed the gel filtration chromatography. A.D.S. helped with mouse experiments. J.F. Reiter wrote the manuscript and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8. (PDF 4500 kb)
Supplementary Table 1
Tctn1-LAP interactors (XLS 201 kb)
Rights and permissions
About this article
Cite this article
Garcia-Gonzalo, F., Corbit, K., Sirerol-Piquer, M. et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43, 776–784 (2011). https://doi.org/10.1038/ng.891
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.891
This article is cited by
-
Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies
Nature Reviews Nephrology (2024)
-
Joubert syndrome causing mutation in C2 domain of CC2D2A affects structural integrity of cilia and cellular signaling molecules
Experimental Brain Research (2024)
-
INPP5E regulates CD3ζ enrichment at the immune synapse by phosphoinositide distribution control
Communications Biology (2023)
-
Primary cilia as dynamic and diverse signalling hubs in development and disease
Nature Reviews Genetics (2023)
-
The tectonic complex regulates membrane protein composition in the photoreceptor cilium
Nature Communications (2023)