Abstract
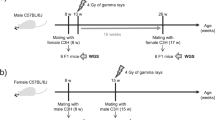
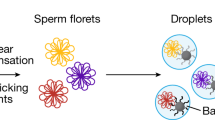
Meiotic recombination between highly similar duplicated sequences (nonallelic homologous recombination, NAHR) generates deletions, duplications, inversions and translocations, and it is responsible for genetic diseases known as 'genomic disorders', most of which are caused by altered copy number of dosage-sensitive genes. NAHR hot spots have been identified within some duplicated sequences. We have developed sperm-based assays to measure the de novo rate of reciprocal deletions and duplications at four NAHR hot spots. We used these assays to dissect the relative rates of NAHR between different pairs of duplicated sequences. We show that (i) these NAHR hot spots are specific to meiosis, (ii) deletions are generated at a higher rate than their reciprocal duplications in the male germline and (iii) some of these genomic disorders are likely to have been underascertained clinically, most notably that resulting from the duplication of 7q11, the reciprocal of the deletion causing Williams-Beuren syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Stankiewicz, P. & Lupski, J.R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 18, 74–82 (2002).
Lupski, J.R. & Stankiewicz, P. Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 1, e49 (2005).
Redon, R. et al. Global variation in copy number in the human genome. Nature 444, 444–454 (2006).
Hurles, M.E. & Lupski, J.R. Recombination hotspots in nonallelic homologous recombination. in Genomic Disorders: the Genomic Basis of Disease Ch. 24 (eds. Lupski, J.R. & Stankiewicz, P.) 341–355 (Humana Press, Totowa, New Jersey, USA, 2006).
Chance, P.F. et al. Two autosomal-dominant neuropathies result from reciprocal DNA duplication/deletion of a region on chromosome-17. Hum. Mol. Genet. 3, 223–228 (1994).
Potocki, L. et al. Molecular mechanism for duplication 17p11.2—the homologous recombination reciprocal of the Smith-Magenis microdeletion. Nat. Genet. 24, 84–87 (2000).
Long, F.L., Duckett, D.P., Billam, L.J., Williams, D.K. & Crolla, J.A. Triplication of 15q11-q13 with inv dup(15) in a female with developmental delay. J. Med. Genet. 35, 425–428 (1998).
Edelmann, L. et al. A common molecular basis for rearrangement disorders on chromosome 22q11. Hum. Mol. Genet. 8, 1157–1167 (1999).
Somerville, M.J. et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N. Engl. J. Med. 353, 1694–1701 (2005).
Bayes, M., Magano, L.F., Rivera, N., Flores, R. & Perez Jurado, L.A. Mutational mechanisms of Williams-Beuren syndrome deletions. Am. J. Hum. Genet. 73, 131–151 (2003).
Blanco, P. et al. Divergent outcomes of intrachromosomal recombination on the human Y chromosome: male infertility and recurrent polymorphism. J. Med. Genet. 37, 752–758 (2000).
Bosch, E. & Jobling, M.A. Duplications of the AZFa region of the human Y chromosome are mediated by homologous recombination between HERVs and are compatible with male fertility. Hum. Mol. Genet. 12, 341–347 (2003).
Reiter, L.T. et al. A recombination hotspot responsible for two inherited peripheral neuropathies is located near a mariner transposon-like element. Nat. Genet. 12, 288–297 (1996).
Shaw, C.J., Withers, M.A. & Lupski, J.R. Uncommon deletions of the Smith-Magenis syndrome region can be recurrent when alternate low-copy repeats act as homologous recombination substrates. Am. J. Hum. Genet. 75, 75–81 (2004).
Jeffreys, A.J., Murray, J. & Neumann, R. High-resolution mapping of crossovers in human sperm defines a minisatellite-associated recombination hotspot. Mol. Cell 2, 267–273 (1998).
Han, L.L., Keller, M.P., Navidi, W., Chance, P.F. & Arnheim, N. Unequal exchange at the Charcot-Marie-Tooth disease type 1A recombination hotspot is not elevated above the genome average rate. Hum. Mol. Genet. 9, 1881–1889 (2000).
Tiemann-Boege, I., Calabrese, P., Cochran, D.M., Sokol, R. & Arnheim, N. High-resolution recombination patterns in a region of human chromosome 21 measured by sperm typing. PLoS Genet. 2, e70 (2006).
Lupski, J. Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. 14, 417–422 (1998).
Lindsay, S.J., Khajavi, M., Lupski, J.R. & Hurles, M.E. A chromosomal rearrangement hotspot can be identified from population genetic variation and is coincident with a hotspot for allelic recombination. Am. J. Hum. Genet. 79, 890–902 (2006).
Osborne, L.R. et al. A 1.5 million-base pair inversion polymorphism in families with Williams-Beuren syndrome. Nat. Genet. 29, 321–325 (2001).
Lopes, J. et al. Fine mapping of de novo CMT1A and HNPP rearrangements within CMT1A-REPs evidences two distinct sex-dependent mechanisms and candidate sequences involved in recombination. Hum. Mol. Genet. 7, 141–148 (1998).
May, C.A., Shone, A.C., Kalaydjieva, L., Sajantila, A. & Jeffreys, A.J. Crossover clustering and rapid decay of linkage disequilibrium in the Xp/Yp pseudoautosomal gene SHOX. Nat. Genet. 31, 272–275 (2002).
Jeffreys, A.J. & Neumann, R. Reciprocal crossover asymmetry and meiotic drive in a human recombination hot spot. Nat. Genet. 31, 267–271 (2002).
Potocki, L. et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am. J. Hum. Genet. 80, 633–649 (2007).
Infante, J. et al. Diagnostic strategy for familial and sporadic cases of neuropathy associated with 17p11.2 deletion. Muscle Nerve 24, 1149–1155 (2001).
Kumar, N., Cole, J. & Parry, G.J. Variability of presentation in hereditary neuropathy with liability to pressure palsy results in underrecognition. Ann. NY Acad. Sci. 883, 344–350 (1999).
Thomas, N.S. et al. Parental and chromosomal origins of microdeletion and duplication syndromes involving 7q11.23, 15q11-q13 and 22q11. Eur. J. Hum. Genet. 14, 831–837 (2006).
Kriek, M. et al. Copy number variation in regions flanked (or unflanked) by duplicons among patients with developmental delay and/or congenital malformations; detection of reciprocal and partial Williams-Beuren duplications. Eur. J. Hum. Genet. 14, 180–189 (2006).
Kirchhoff, M., Bisgaard, A.M., Bryndorf, T. & Gerdes, T. MLPA analysis for a panel of syndromes with mental retardation reveals imbalances in 5.8% of patients with mental retardation and dysmorphic features, including duplications of the Sotos syndrome and Williams-Beuren syndrome regions. Eur. J. Med. Genet. 50, 33–42 (2007).
Torniero, C. et al. Cortical dysplasia of the left temporal lobe might explain severe expressive-language delay in patients with duplication of the Williams-Beuren locus. Eur. J. Hum. Genet. 15, 62–67 (2007).
Kent, W.J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Fiegler, H. et al. Accurate and reliable high-throughput detection of copy number variation in the human genome. Genome Res. 16, 1566–1574 (2006).
Acknowledgements
The authors are grateful to L. Osborne for information on WBS-LCR duplications, C. May for advice on sperm recombination assays, and J. Lupski and A. Jeffreys for comments on an earlier version of the manuscript. We would like to thank the sperm donors themselves and the staff at the Bourn Clinic. This work was funded by the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
M.E.H. and D.J.T. designed this study, M.L.B., S.B. and M.M. performed sample collection, and N.P.C., H.F. and D.R. carried out array–CGH experiments. All other experiments were performed by D.J.T. M.E.H. and D.J.T. performed the analysis and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Methods, Supplementary Figures 1–3, Supplementary Tables 1–3 (PDF 1227 kb)
Rights and permissions
About this article
Cite this article
Turner, D., Miretti, M., Rajan, D. et al. Germline rates of de novo meiotic deletions and duplications causing several genomic disorders. Nat Genet 40, 90–95 (2008). https://doi.org/10.1038/ng.2007.40
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2007.40
This article is cited by
-
Read-depth based approach on whole genome resequencing data reveals important insights into the copy number variation (CNV) map of major global buffalo breeds
BMC Genomics (2023)
-
Sequencing individual genomes with recurrent genomic disorder deletions: an approach to characterize genes for autosomal recessive rare disease traits
Genome Medicine (2022)
-
Genomic structural variation in tomato and its role in plant immunity
Molecular Horticulture (2022)
-
Diagnosis of prenatal 22q11.2 duplication syndrome: a two-case study
Journal of Genetics (2022)
-
Copy number variations of chromosome 17p11.2 region in children with development delay and in fetuses with abnormal imaging findings
BMC Medical Genomics (2021)