Abstract
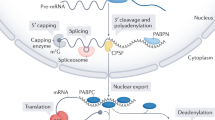
About half of human genes use alternative cleavage and polyadenylation (ApA) to generate messenger RNA transcripts that differ in the length of their 3′ untranslated regions (3′ UTRs) while producing the same protein1,2,3. Here we show in human cell lines that alternative 3′ UTRs differentially regulate the localization of membrane proteins. The long 3′ UTR of CD47 enables efficient cell surface expression of CD47 protein, whereas the short 3′ UTR primarily localizes CD47 protein to the endoplasmic reticulum. CD47 protein localization occurs post-translationally and independently of RNA localization. In our model of 3′ UTR-dependent protein localization, the long 3′ UTR of CD47 acts as a scaffold to recruit a protein complex containing the RNA-binding protein HuR (also known as ELAVL1) and SET4 to the site of translation. This facilitates interaction of SET with the newly translated cytoplasmic domains of CD47 and results in subsequent translocation of CD47 to the plasma membrane via activated RAC1 (ref. 5). We also show that CD47 protein has different functions depending on whether it was generated by the short or long 3′ UTR isoforms. Thus, ApA contributes to the functional diversity of the proteome without changing the amino acid sequence. 3′ UTR-dependent protein localization has the potential to be a widespread trafficking mechanism for membrane proteins because HuR binds to thousands of mRNAs6,7,8,9, and we show that the long 3′ UTRs of CD44, ITGA1 and TNFRSF13C, which are bound by HuR, increase surface protein expression compared to their corresponding short 3′ UTRs. We propose that during translation the scaffold function of 3′ UTRs facilitates binding of proteins to nascent proteins to direct their transport or function—and this role of 3′ UTRs can be regulated by ApA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sandberg, R., Neilson, J. R., Sarma, A., Sharp, P. A. & Burge, C. B. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320, 1643–1647 (2008)
Mayr, C. & Bartel, D. P. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138, 673–684 (2009)
Lianoglou, S., Garg, V., Yang, J. L., Leslie, C. S. & Mayr, C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes Dev. 27, 2380–2396 (2013)
Brennan, C. M., Gallouzi, I. E. & Steitz, J. A. Protein ligands to HuR modulate its interaction with target mRNAs in vivo . J. Cell Biol. 151, 1–14 (2000)
ten Klooster, J. P., Leeuwen, I., Scheres, N., Anthony, E. C. & Hordijk, P. L. Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET. EMBO J. 26, 336–345 (2007)
Kishore, S. et al. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nature Methods 8, 559–564 (2011)
Lebedeva, S. et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell 43, 340–352 (2011)
Mukherjee, N. et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell 43, 327–339 (2011)
Uren, P. J. et al. Genomic analyses of the RNA-binding protein Hu antigen R (HuR) identify a complex network of target genes and novel characteristics of its binding sites. J. Biol. Chem. 286, 37063–37066 (2011)
An, J. J. et al. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134, 175–187 (2008)
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000)
Jaiswal, S. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 (2009)
Fan, X. C. & Steitz, J. A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 17, 3448–3460 (1998)
Mazan-Mamczarz, K. et al. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc. Natl Acad. Sci. USA 100, 8354–8359 (2003)
Seo, S. B. et al. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell 104, 119–130 (2001)
Fan, Z., Beresford, P. J., Oh, D. Y., Zhang, D. & Lieberman, J. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell 112, 659–672 (2003)
Miller, J. D., Wilhelm, H., Gierasch, L., Gilmore, R. & Walter, P. GTP binding and hydrolysis by the signal recognition particle during initiation of protein translocation. Nature 366, 351–354 (1993)
Schneider, R., Bannister, A. J., Weise, C. & Kouzarides, T. Direct binding of INHAT to H3 tails disrupted by modifications. J. Biol. Chem. 279, 23859–23862 (2004)
Zerangue, N., Schwappach, B., Jan, Y. N. & Jan, L. Y. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22, 537–548 (1999)
Walmsley, M. J. et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science 302, 459–462 (2003)
Reinhold, M. I., Green, J. M., Lindberg, F. P., Ticchioni, M. & Brown, E. J. Cell spreading distinguishes the mechanism of augmentation of T cell activation by integrin-associated protein/CD47 and CD28. Int. Immunol. 11, 707–718 (1999)
Lamy, L. et al. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J. Biol. Chem. 278, 23915–23921 (2003)
Lindberg, F. P. et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274, 795–798 (1996)
Isenberg, J. S. et al. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am. J. Pathol. 173, 1100–1112 (2008)
Soto-Pantoja, D. R., Isenberg, J. S. & Roberts, D. D. Therapeutic targeting of CD47 to modulate tissue responses to ischemia and radiation. J. Genet. Syndr. Gene Ther. 2, 1000105 (2011)
Frazier, W. A., Isenberg, J. S., Kaur, S. & Roberts, D. D. in UCSD Nature Molecule Pages (University of California, San Diego, 2010)
Avet, C. et al. SET protein interacts with intracellular domains of the gonadotropin-releasing hormone receptor and differentially regulates receptor signaling to cAMP and calcium in gonadotrope cells. J. Biol. Chem. 288, 2641–2654 (2013)
Nilsson, J., Persson, B. & von Heijne, G. Comparative analysis of amino acid distributions in integral membrane proteins from 107 genomes. Proteins 60, 606–616 (2005)
Lau, A. G. et al. Distinct 3′UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Natl Acad. Sci. USA 107, 15945–15950 (2010)
Yoon, J. H. et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nature Commun. 4, 2939 (2013)
Neviani, P. et al. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 8, 355–368 (2005)
Nho, R. S. et al. PTEN regulates fibroblast elimination during collagen matrix contraction. J. Biol. Chem. 281, 33291–33301 (2006)
Reinhold, M. I. et al. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J. Cell Sci. 108, 3419–3425 (1995)
Slobodin, B. & Gerst, J. E. A novel mRNA affinity purification technique for the identification of interacting proteins and transcripts in ribonucleoprotein complexes. RNA 16, 2277–2290 (2010)
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998)
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992)
Niranjanakumari, S., Lasda, E., Brazas, R. & Garcia-Blanco, M. A. Reversible cross-linking combined with immunoprecipitation to study RNA–protein interactions in vivo . Methods 26, 182–190 (2002)
Mili, S. & Steitz, J. A. Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA 10, 1692–1694 (2004)
Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4, 44–57 (2009)
Stagljar, I. & Fields, S. Analysis of membrane protein interactions using yeast-based technologies. Trends Biochem. Sci. 27, 559–563 (2002)
Acknowledgements
This work was funded by the Innovator Award of the Damon Runyon-Rachleff Cancer Foundation and the Island Outreach Foundation (DRR-24-13) and National Institutes of Health grant U01-CA164190. We thank N. Patel for help with cloning of the constructs and the members of the Mayr laboratory, specifically S.-H. Lee and E. Kallin, as well as N. Rajewsky and A. Ventura, for helpful discussions. We also thank J. Joyce, C. Haynes, A. Hall and R. Benezra for critical reading of the manuscript. The N17RAC1 construct was provided by A. Hall and the JinB8 cells by W. A. Frazier and D. D.Roberts. We thank the Molecular Cytology Core Facility (Memorial Sloan Kettering Cancer Center) for help with the confocal microscopy (funded by P30 CA008748).
Author information
Authors and Affiliations
Contributions
B.D.B. designed and performed the experiments and C.M. designed the study. B.D.B. and C.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper. Correspondence and requests for materials should be addressed to C.M. (mayrc@mskcc.org).
Extended data figures and tables
Extended Data Figure 1 Expression of the long CD47 3′ UTR isoform correlates with cell surface expression of CD47 protein.
a, Fluorescence confocal microscopy of cells shown as in Fig. 1a. Representative images out of hundreds of cells are shown. Scale bars, 10 µm. b, FACS analysis of endogenous CD47 expression in cells shown in Fig. 1a and a. Permeabilized cells show total CD47 expression (purple) and non-permeabilized cells show surface CD47 expression (blue). Representative histograms are shown (HEK293 cells, n = 10; U2OS, Jurkat cells, n = 5; Caov-3, n = 3). Unstained cells are shown in grey. c, Left, quantification of mean fluorescence intensity (MFI) values from b. Right, fraction of surface and intracellular CD47 levels in cells lines from b. Intracellular CD47 was calculated by subtracting CD47 surface values from total CD47 values. d, Northern blot of HEK293 cells stably expressing the indicated shRNAs and hybridized for CD47. The shRNAs against CD47-LU target only the long 3′ UTR isoforms of CD47. The blot and corresponding RNA gel are shown as in Fig. 1c. e, Quantification of CD47 total mRNA and 3′ UTR isoform levels in U2OS cells by qRT–PCR. GAPDH-normalized values after transfection of sh2 CD47-LU or sh Co are shown as the mean ± s.d., n = 3 biological replicates. The total amount of CD47 mRNA after transfection of sh Co was set to 1. f, FACS analysis of endogenous CD47 protein expression after stable expression of shRNAs against CD47-LU in HEK293 cells. Surface (top) and total (bottom) CD47 expression is shown. Representative histograms out of n = 3 experiments are shown. Unstained cells are shown in grey. g, Quantification of MFI values from f is displayed. Intracellular CD47 was calculated as in b. h, Northern blot of HEK293 cells after transfection of indicated constructs and hybridized against CD47. Mutation of the proximal polyadenylation signal in CD47-LU abrogates production of short 3′ UTR isoforms. Asterisk indicates cross-hybridization to ribosomal RNAs. i, Fluorescence confocal microscopy of endogenous CD47 and calnexin protein in permeabilized U2OS cells. Calnexin partially co-localizes with CD47. A representative image out of hundreds of cells is shown. Scale bars, 10 µm.
Extended Data Figure 2 UDPL depends on HuR, SET and RAC1 and mediates surface localization of membrane proteins.
a, Western blot of HEK293 cells transiently transfected (left, middle) or stably expressing (right) sh Co or shRNAs against HuR. The blot shows reduced HuR protein expression after HuR knockdown, but no change in protein expression of CD47, TSPAN13, CD44 or SET. Actin was used as loading control. b, Quantification of CD47 total mRNA and 3′ UTR isoform levels in HEK293 cells by qRT–PCR. GAPDH-normalized values after transfection of sh2 HuR or sh Co are shown. Shown is the mean ± s.d., n = 3 biological replicates. The total amount of CD47 mRNA after transfection of sh Co was set to 1. c, FACS analysis of HEK293 cells stably expressing the indicated shRNAs. Histograms are shown as in Fig. 2b. Representative histograms from n = 3 experiments are shown. d, Western blot of HEK293 cells stably expressing shRNAs against SET. Actin was used as loading control. The marker is shown in kDa. e, As in d, but HEK293 cells stably expressing shRNAs against RAC1 are shown. f, 3′-seq analysis shows 3′ UTR isoform expression of ITGA1 in B-LCL and TSPAN13 in HEK293 cells shown as in Fig. 1b. FACS analysis of endogenous protein levels is shown as in Fig. 2c. Left panel shows ITGA1 expression in HeLa cells and right panel shows TSPAN13 expression in HEK293 cells. Representative histograms from n = 2 experiments are shown. g, FACS analysis of GFP after transfection of constructs containing a signal peptide and GFP fused to the TMD, C terminus and either the long 3′ UTR (dark blue line) or the short 3′ UTR (light blue line) of ITGA1 in HEK293 cells. Representative histograms from n = 3 experiments are shown as in Fig. 2d.
Extended Data Figure 3 3′ UTR isoforms that encode proteins using UDPL contain uridine-rich elements.
Shown are the 3′ UTR sequences of CD47, CD44, HuR-BS and HuR-BSΔ. Red, ApA signals. Blue, uridine-rich elements with the potential to be HuR-binding sites.
Extended Data Figure 4 3′ UTR isoforms that encode proteins using UDPL contain uridine-rich elements.
Shown are the 3′ UTR sequences of ITGA1, TNFRSF13C and TSPAN13. Red, ApA signals. Blue, uridine-rich elements with the potential to be HuR-binding sites.
Extended Data Figure 5 Local recruitment of SET to the site of translation is required for UDPL.
a, Western blot of cells used in Fig. 3b shows the amount of overexpression achieved by transfection of MS2-mC-SET or MS2-mC-HuR (for constructs, see b). Left, anti-SET detects endogenous expression of SET as well as overexpressed SET. Right, anti-HuR detects endogenous HuR and overexpressed HuR. Actin was used as loading control. Anti-HuR and anti-SET were used on the same blot. Actin as loading control was performed once. The marker is shown in kDa. Asterisk indicates unspecific band. mC, mCherry. b, The top construct depicts GFP-TM-SU (Fig. 1e) and the bottom construct shows a fusion of MS2 coat protein (MS2), mC (red) and HuR or SET, respectively. Overexpression of HuR or SET compared with expression of MS2-mC alone does not change surface or total GFP expression, when co-transfected with GFP-TM-SU (without the addition of MS2-binding sites to the SU isoform) as shown by FACS analysis. Surface expression (top) and total expression (bottom) in HEK293 cells are shown. Values for MFI are shown in parentheses. Unstained cells are shown in grey. Representative histograms from n = 2 experiments are shown. c, FACS analysis of cells used in Fig. 3b. MS2-binding sites (MS2-BS, RNA stem loops) were added to GFP-TM-SU (and the proximal polyadenylation signal was mutated) to obtain GFP-TM-SU-MS2-BS. Transfection of MS2-mC-HuR (left, dark purple line) or MS2-mC-SET (right, dark purple line) increases surface GFP expression compared with transfection of MS2-mC (light purple line), when GFP-TM-SU-MS2-BS is co-transfected. Thus, tethering of HuR or SET to the short 3′ UTR of GFP-TM localizes GFP to the cell surface without changing total GFP expression. Histograms are shown as in b. Representative histograms from n = 5 experiments are shown. d, As in c, but tethering was impaired by omission of the MS2 coat protein. Histograms are shown as in b. Representative histograms from n = 2 experiments are shown. Summary of the tethering experiment: To tether SET or HuR to the 3′ UTR (which brings it close to the site of translation through the scaffold function of the 3′ UTR), we added MS2-binding sites to GFP-TM-SU c, MS2-binding sites are derived from the bacteriophage MS2 and form RNA stem loops. The capsid protein of MS2 (here, called MS2) specifically recognizes these MS2 stem loops. Constructs were generated containing MS2 fused to mC and then either HuR, SET or with no further coding sequence (Fig. 3b). Co-expression of these constructs with the construct containing the short UTR and MS2-binding sites results in recruitment of SET or HuR to the short 3′ UTR of GFP-TM. The cells that express MS2 fused to only mC localize GFP to the endoplasmic reticulum, but constructs containing MS2 fusions to HuR or SET localize GFP primarily to the cell surface (Fig. 3b and Extended Data Fig. 5c). Omitting either MS2 or the MS2-binding sites from the experiment abrogates surface localization (Extended Data Fig. 5b, d).
Extended Data Figure 6 CD47 contains at least two SET-binding sites in its cytoplasmic domains.
a, FACS analysis of surface GFP expression after transfection of GFP-TM-LU (dark blue line) and GFP-TM-LU constructs containing a single point mutation in the cytoplasmic C terminus (light blue line), K290A (left), K297A (middle), K304A (right) in HEK293 cells. Partial destruction of a single SET-binding site results in up to 37% reduction in GFP surface expression. Values for MFI are shown in parentheses. Unstained cells are shown in grey. Representative histograms from n = 5 experiments are shown. b, FACS analysis of GFP expression after transfection of GFP-TM-LU (dark blue line), GFP-TM-LU containing a mutation of the SET-binding site in the C terminus (K290A, K304A; 2Km; light blue line; left), containing a deletion of the C terminus (ΔC; light blue line; middle panel), or destruction of both SET-binding sites (ΔC combined with K163A, K166A, K175A; ΔCL; light blue line; right). Surface (top) and total (bottom) expression is shown in HEK293 cells. Values for MFI are shown in parentheses. Unstained cells are shown in grey. Representative histograms from several experiments are shown (2Km, n = 3; ΔC, n = 10; ΔCL, n = 4).
Extended Data Figure 7 CD47 protein has different functions depending on whether it was generated by the SU or LU isoform.
a, Left, western blot of HEK293 cells after transfection of the indicated constructs shows GFP–CD47 expression using an anti-GFP antibody. Actin was used as loading control. Right, as in left panel after transfection of CD47-SU and CD47-LU into HEK293 (left) or JinB8 cells (right). GFP–CD47 expression was quantified after normalization with respect to actin using Image J. Shown is the fold change in GFP–CD47 expression of CD47-SU after setting CD47-LU to 1. b, The experiment is similar to Fig. 3b and Extended Data Fig. 5c, but here the constructs containing the full open reading frame of CD47 were used. FACS analysis of GFP expression after transfection of CD47-SU-MS2-BS. Co-transfection of MS2-mC-HuR (left, dark purple line) or MS2-mC-SET (right, dark purple line) increases surface GFP expression compared to co-transfection of MS2-mC (light purple line). Surface expression is shown in non-permeabilized HEK293 cells. Values for MFI are shown in parentheses. Representative histograms from n = 3 experiments are shown. Unstained cells are shown in grey. c, Left, FACS analysis of GFP after transfection of constructs containing a signal peptide and GFP fused to the open reading frame of BAFFR and either the long 3′ UTR (BAFFR-LU, dark blue line) or the short 3′ UTR (BAFFR-SU, light blue line) in HEK293 cells. Surface (top) and total (bottom) GFP expression is shown. Values for MFI are shown in parentheses. Representative histograms from n = 3 experiments are shown. Unstained cells are shown in grey. Right, as in left panel but for CD44. d, Table showing the fold increase in surface GFP expression mediated by the LU isoform compared with the SU isoform. Top row shows values of constructs without the ECD and bottom row shows values of constructs containing the full coding regions of the indicated proteins. The fold increase in surface GFP expression was calculated from MFI (LU)/MFI (SU). The contribution of the ECD domain for surface expression of BAFFR is 1.2-fold (3.8/3.1). e, FACS analysis of carboxyfluorescein succinimidyl ester (CFSE) uptake in macrophages. Macrophages were co-cultured without (grey) cells or with cells that were pre-treated with CFSE and expressed high or low amounts of surface CD47 (data not shown). The experiment shows that the macrophages phagocytose the cells depending on their CD47 surface expression levels. A representative histogram from n = 2 experiments is shown. f, The fraction of surviving cells (TO-PRO3 negative) as measured by FACS analysis at day 3 (d3) after increasing doses of γ-irradiation is shown for Jurkat, JinB8 (CD47−/− ) and the GFP+ fraction after nucleofection of JinB8 cells with either CD47-SU or CD47-LU. The values were obtained from the same experiment as shown in Fig. 4e, but here the values were calculated using all GFP-positive cells. Shown are the values for mean ± s.d., n = 3 biological replicates. Gy, Gray. g, The fraction of surviving cells (TO-PRO3 negative) as measured by FACS analysis at day 3 (d3) after increasing doses of γ-irradiation is shown for Jurkat, JinB8 (CD47−/− ) and the GFP+ fraction after nucleofection of Jurkat cells with sh2 CD47-LU. Shown are the 20% of cells with the highest GFP expression (green). Shown are the values for mean ± s.d., n = 3 biological replicates. Gy, Gray.
Extended Data Figure 8 HuR, SET and RAC1 are widely and highly expressed.
a, The mRNAs of proteins necessary for UDPL are ubiquitously and highly expressed across cell lines (left) and tissues (right). Shown are values for transcripts per million (TPM). The median abundance levels of all expressed genes in the data sets are shown as dashed lines. ELAVL1 encodes HuR. The data set from ref. 3 was analysed to obtain the TPM values. b, Here, ‘HuR targets’ consist of the union of HuR targets identified previously7,9. Membrane proteins consist of all the proteins that contain the tag “membrane” using gene ontology analysis. The fraction of membrane proteins found is consistent with the fraction of membrane proteins found in yeast40. Fisher’s exact test shows no enrichment or depletion of membrane proteins among the HuR targets.
Extended Data Figure 9 All tested UDPL candidates have potential SET-binding sites in their cytoplasmic domains.
Shown are the amino acid sequences of the TMDs and cytoplasmic domains of the membrane proteins studied. The TMDs are shown in green and the positively charged amino acids in the cytoplasmic domains, indicating potential SET-binding sites, are shown in red.
Rights and permissions
About this article
Cite this article
Berkovits, B., Mayr, C. Alternative 3′ UTRs act as scaffolds to regulate membrane protein localization. Nature 522, 363–367 (2015). https://doi.org/10.1038/nature14321
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14321
This article is cited by
-
Application of genome editing techniques to regulate gene expression in crops
BMC Plant Biology (2024)
-
Multiplexed screening reveals how cancer-specific alternative polyadenylation shapes tumor growth in vivo
Nature Communications (2024)
-
Transcriptomics-proteomics Integration reveals alternative polyadenylation driving inflammation-related protein translation in patients with diabetic nephropathy
Journal of Translational Medicine (2023)
-
mRNA nanodelivery systems: targeting strategies and administration routes
Biomaterials Research (2023)
-
mRNA 3’UTR lengthening by alternative polyadenylation attenuates inflammatory responses and correlates with virulence of Influenza A virus
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.