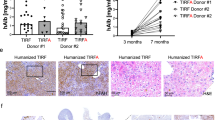
Abstract
Recombinant adeno-associated viral (rAAV) vectors have shown early promise in clinical trials1,2,3. The therapeutic transgene cassette can be packaged in different AAV capsid pseudotypes, each having a unique transduction profile. At present, rAAV capsid serotype selection for a specific clinical trial is based on effectiveness in animal models. However, preclinical animal studies are not always predictive of human outcome4,5,6,7,8. Here, in an attempt to further our understanding of these discrepancies, we used a chimaeric human–murine liver model to compare directly the relative efficiency of rAAV transduction in human versus mouse hepatocytes in vivo. As predicted from preclinical and clinical studies4,5,8, rAAV2 vectors functionally transduced mouse and human hepatocytes at equivalent but relatively low levels. However, rAAV8 vectors, which are very effective in many animal models, transduced human hepatocytes rather poorly—approximately 20 times less efficiently than mouse hepatocytes. In light of the limitations of the rAAV vectors currently used in clinical studies, we used the same murine chimaeric liver model to perform serial selection using a human-specific replication-competent viral library composed of DNA-shuffled AAV capsids. One chimaeric capsid composed of five different parental AAV capsids was found to transduce human primary hepatocytes at high efficiency in vitro and in vivo, and provided species-selected transduction in primary liver, cultured cells and a hepatocellular carcinoma xenograft model. This vector is an ideal clinical candidate and a reagent for gene modification of human xenotransplants in mouse models of human diseases. More importantly, our results suggest that humanized murine models may represent a more precise approach for both selecting and evaluating clinically relevant rAAV serotypes for gene therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
10 January 2014
Changes were made to the keys of Fig. 3c, e.
References
Bainbridge, J. W. et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 (2008)
Gaudet, D. et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 20, 361–369 (2013)
Bennett, J. et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci. Trans. Med. 4, 120ra115 (2012)
Nietupski, J. B. et al. Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression. Mol. Ther. 19, 1999–2011 (2011)
Jiang, H. et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 108, 3321–3328 (2006)
Nathwani, A. C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011)
Kay, M. A. et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nature Genet. 24, 257–261 (2000)
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature Med. 12, 342–347 (2006)
Nathwani, A. C. et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood 109, 1414–1421 (2007)
Nathwani, A. C. et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 107, 2653–2661 (2006)
Davidoff, A. M. et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol. Ther. 11, 875–888 (2005)
Nonnenmacher, M. & Weber, T. Intracellular transport of recombinant adeno-associated virus vectors. Gene Ther. 19, 649–658 (2012)
Azuma, H. et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nature Biotechnol. 25, 903–910 (2007)
Grimm, D. et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 82, 5887–5911 (2008)
Müller, O. J. et al. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nature Biotechnol. 21, 1040–1046 (2003)
Perabo, L. et al. In vitro selection of viral vectors with modified tropism: the adeno-associated virus display. Mol. Ther. 8, 151–157 (2003)
Maheshri, N., Koerber, J. T., Kaspar, B. K. & Schaffer, D. V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nature Biotechnol. 24, 198–204 (2006)
Li, W. et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol. Ther. 16, 1252–1260 (2008)
Pulicherla, N. et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 19, 1070–1078 (2011)
Asuri, P. et al. Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol. Ther. 20, 329–338 (2012)
Yang, L. et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc. Natl Acad. Sci. USA 106, 3946–3951 (2009)
Choi, V. W., McCarty, D. M. & Samulski, R. J. AAV hybrid serotypes: improved vectors for gene delivery. Curr. Gene Ther. 5, 299–310 (2005)
Rutledge, E. A., Halbert, C. L. & Russell, D. W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72, 309–319 (1998)
Petek, L. M., Fleckman, P. & Miller, D. G. Efficient KRT14 targeting and functional characterization of transplanted human keratinocytes for the treatment of epidermolysis bullosa simplex. Mol. Ther. 18, 1624–1632 (2010)
Calcedo, R., Vandenberghe, L. H., Gao, G., Lin, J. & Wilson, J. M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 199, 381–390 (2009)
Boutin, S. et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 (2010)
Ling, C. et al. Human hepatocyte growth factor receptor is a cellular coreceptor for adeno-associated virus serotype 3. Hum. Gene Ther. 21, 1741–1747 (2010)
Hazari, S. et al. Hepatocellular carcinoma xenograft supports HCV replication: a mouse model for evaluating antivirals. World J. Gastroenterol. 17, 300–312 (2011)
Thomas, C. E., Storm, T. A., Huang, Z. & Kay, M. A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 78, 3110–3122 (2004)
Cunningham, S. C., Dane, A. P., Spinoulas, A., Logan, G. J. & Alexander, I. E. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol. Ther. 16, 1081–1088 (2008)
Nakai, H. et al. A limited number of transducible hepatocytes restricts a wide-range linear vector dose response in recombinant adeno-associated virus-mediated liver transduction. J. Virol. 76, 11343–11349 (2002)
Grompe, M. & Strom, S. Mice with human livers. Gastroenterology (2013)
Lieber, A., Peeters, M. J., Gown, A., Perkins, J. & Kay, M. A. A modified urokinase plasminogen activator induces liver regeneration without bleeding. Hum. Gene Ther. 6, 1029–1037 (1995)
Arbetman, A. E. et al. Novel caprine adeno-associated virus (AAV) capsid (AAV-Go.1) is closely related to the primate AAV-5 and has unique tropism and neutralization properties. J. Virol. 79, 15238–15245 (2005)
Lisowski, L. et al. Ribosomal DNA integrating rAAV-rDNA vectors allow for stable transgene expression. Mol. Ther. 20, 1912–1923 (2012)
Acknowledgements
This work was supported by National Institutes of Health grants HL092096 and HL064274 to M.A.K. and DK048252 to M.G.; L.L. was supported in part by the Berry Fellowship Foundation; I.E.A. by Australian National Health and Medical Research Council (NHMRC) grant 1008021.
Author information
Authors and Affiliations
Contributions
L.L. helped with study design, performed experiments and data analysis, prepared figures and the manuscript. A.D. performed some of the experiments and data analysis, and assisted in figure preparation and manuscript editing. K.C. helped in performing some of the experiments. Y.Z. performed some of the vector sequence analysis. S.C.C. performed some of the animal studies and assisted in manuscript editing. E.M.W. generated the human transplanted FRG mice in Fig. 4c. S.N. injected the animals and prepared tissues for the experiment in Fig. 4c. M.G. helped with establishing the FRG colony and provided advice on in vivo human hepatocyte repopulation. I.E.A. helped with study design and manuscript editing. M.A.K. helped with study coordination, manuscript writing and editing. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
Oregon Health and Science University (OHSU) and M.G. have a significant financial interest in Yecuris Corp., a company that has some commercial interests in the FRG mouse. M.A.K. has a minor equity stake with stock options value >US$5,000 and has no role in the company. E.M.W. is an employee of Yecuris and has no equity. M.G. and Yecuris have no ownership or intellectual property rights to any of the new AAV vectors described herein including AAV-LK03.
Extended data figures and tables
Extended Data Figure 1 IVIG neutralization assay optimization on Huh-7 cells using rAAV2-RSV-Luc2.
Gamunex and Gammagard IVIGs were compared at two different temperatures. See Methods for experimental details.
Extended Data Figure 2 In vivo vectors comparison in C57/BL6 animals.
a, b, In vivo average VCN analysis in tissues harvested on day 54 (a) from the first in vivo rAAV-hFIX experiment and on day 7 (b) from the second in vivo rAAV-hFIX experiment. c, In vivo hFIX expression levels. hFIX levels obtained from the first in vivo rAAV-hFIX comparison (solid colour lines, from day 5 until day 80) are presented on the same graph with data obtained during the second in vivo experiment (dotted lines, days 2, 4 and 7).
Extended Data Figure 3 Time course of Luc signal in animals shown in Fig. 4a.
a, b, Data for days 2, 4 and 6 were collected and are shown. In a, all animals are shown with the same pseudo-scale, whereas in b, auto-scale was selected for each group.
Extended Data Figure 4 Detailed analysis of bioluminescence for animals shown in Fig. 4b.
The table represents detailed information on signal for each animal/ROI.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Figures 1-4. (PDF 5149 kb)
Rights and permissions
About this article
Cite this article
Lisowski, L., Dane, A., Chu, K. et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014). https://doi.org/10.1038/nature12875
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12875
This article is cited by
-
Efficient and safe therapeutic use of paired Cas9-nickases for primary hyperoxaluria type 1
EMBO Molecular Medicine (2024)
-
A humanized mouse model for adeno-associated viral gene therapy
Nature Communications (2024)
-
The AAV capsid can influence the epigenetic marking of rAAV delivered episomal genomes in a species dependent manner
Nature Communications (2023)
-
tRNA therapeutics for genetic diseases
Nature Reviews Drug Discovery (2023)
-
Cell and gene therapy for kidney disease
Nature Reviews Nephrology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.