Abstract
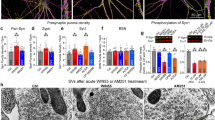
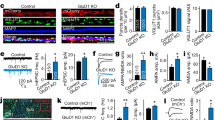
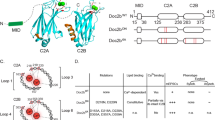
Synaptic neurotransmitter release is driven by Ca2+ influx through active zone voltage-gated calcium channels (VGCCs)1,2. Control of active zone VGCC abundance and function remains poorly understood. Here we show that a trafficking step probably sets synaptic VGCC levels in rats, because overexpression of the pore-forming α1A VGCC subunit fails to change synaptic VGCC abundance or function. α2δs are a family of glycosylphosphatidylinositol (GPI)-anchored VGCC-associated subunits3 that, in addition to being the target of the potent neuropathic analgesics gabapentin and pregabalin (α2δ-1 and α2δ-2)4,5, were also identified in a forward genetic screen for pain genes (α2δ-3)6. We show that these proteins confer powerful modulation of presynaptic function through two distinct molecular mechanisms. First, α2δ subunits set synaptic VGCC abundance, as predicted from their chaperone-like function when expressed in non-neuronal cells3,7. Second, α2δs configure synaptic VGCCs to drive exocytosis through an extracellular metal ion-dependent adhesion site (MIDAS), a conserved set of amino acids within the predicted von Willebrand A domain of α2δ. Expression of α2δ with an intact MIDAS motif leads to an 80% increase in release probability, while simultaneously protecting exocytosis from blockade by an intracellular Ca2+ chelator. α2δs harbouring MIDAS site mutations still drive synaptic accumulation of VGCCs; however, they no longer change release probability or sensitivity to intracellular Ca2+ chelators. Our data reveal dual functionality of these clinically important VGCC subunits, allowing synapses to make more efficient use of Ca2+ entry to drive neurotransmitter release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Llinás, R., Steinberg, I. Z. & Walton, K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc. Natl Acad. Sci. USA 73, 2918–2922 (1976)
Neher, E. & Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59, 861–872 (2008)
Davies, A. et al. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc. Natl Acad. Sci. USA 107, 1654–1659 (2010)
Field, M. J. et al. Identification of the α2-δ-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc. Natl Acad. Sci. USA 103, 17537–17542 (2006)
Wang, M., Offord, J., Oxender, D. L. & Su, T. Z. Structural requirement of the calcium-channel subunit α2δ for gabapentin binding. Biochem. J. 342, 313–320 (1999)
Neely, G. G. et al. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell 143, 628–638 (2010)
Gao, B. et al. Functional properties of a new voltage-dependent calcium channel α2δ auxiliary subunit gene (CACNA2D2). J. Biol. Chem. 275, 12237–12242 (2000)
Arikkath, J. & Campbell, K. P. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 13, 298–307 (2003)
Catterall, W. A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 (2000)
Dunlap, K., Luebke, J. I. & Turner, T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 18, 89–98 (1995)
Cao, Y. Q. et al. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron 43, 387–400 (2004)
Watschinger, K. et al. Functional properties and modulation of extracellular epitope-tagged CaV2.1 voltage-gated calcium channels. Channels 2, 461–473 (2008)
Winterfield, J. R. & Swartz, K. J. A hot spot for the interaction of gating modifier toxins with voltage-dependent ion channels. J. Gen. Physiol. 116, 637–644 (2000)
Dolphin, A. C. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 19, 237–244 (2009)
Pragnell, M. et al. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature 368, 67–70 (1994)
Schneggenburger, R., Sakaba, T. & Neher, E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 25, 206–212 (2002)
Ariel, P. & Ryan, T. A. Optical mapping of release properties in synapses. Front. Neural Circuits 4, 18 (2010)
Catterall, W. A. & Few, A. P. Calcium channel regulation and presynaptic plasticity. Neuron 59, 882–901 (2008)
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods 6, 875–881 (2009)
Dodge, F. A., Jr & Rahamimoff, R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. (Lond.) 193, 419–432 (1967)
Parekh, A. B. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J. Physiol. (Lond.) 586, 3043–3054 (2008)
Cantí, C. et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of α2δ subunits is key to trafficking voltage-gated Ca2+ channels. Proc. Natl Acad. Sci. USA 102, 11230–11235 (2005)
Springer, T. A. Complement and the multifaceted functions of VWA and integrin I domains. Structure 14, 1611–1616 (2006)
Lacy, D. B., Wigelsworth, D. J., Scobie, H. M., Young, J. A. & Collier, R. J. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc. Natl Acad. Sci. USA 101, 6367–6372 (2004)
Whittaker, C. A. & Hynes, R. O. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13, 3369–3387 (2002)
Davies, A. et al. The calcium channel α2δ-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J. Neurosci. 26, 8748–8757 (2006)
Brown, J. T. & Randall, A. Gabapentin fails to alter P/Q-type Ca2+ channel-mediated synaptic transmission in the hippocampus in vitro. Synapse 55, 262–269 (2005)
Tran-Van-Minh, A. & Dolphin, A. C. The α2δ ligand gabapentin inhibits the Rab11-dependent recycling of the calcium channel subunit α2δ-2. J. Neurosci. 30, 12856–12867 (2010)
Kurshan, P. T., Oztan, A. & Schwarz, T. L. Presynaptic α2δ-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nature Neurosci. 12, 1415–1423 (2009)
Kim, S. H. & Ryan, T. A. CDK5 serves as a major control point in neurotransmitter release. Neuron 67, 797–809 (2010)
Miesenböck, G., De Angelis, D. A. & Rothman, J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394, 192–195 (1998)
Voglmaier, S. M. et al. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71–84 (2006)
Shaner, N. C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nature Methods 5, 545–551 (2008)
Viard, P. et al. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nature Neurosci. 7, 939–946 (2004)
Acknowledgements
We thank W. Pratt for performing mutagenesis on α2δ-1 and M. D’Arco for advice on immunocytochemistry. We thank S. Kim for designing the vGmOr2 and VAMPmCh plasmids, P. Ariel for helpful discussion and analysis of exocytosis data, R. Kwan and Y. Gera for technical assistance in cell culture, G. Obermair for providing the wild-type EGFP–α1A plasmid and L. Looger for providing the GCaMP3 plasmid and helpful communication.
Author information
Authors and Affiliations
Contributions
M.B.H. performed opto-physiological experiments, B.L. and W.M. performed electrophysiological experiments, A.C.D., B.L. and W.M. designed electrophysiological experiments, M.B.H., A.C.D. and T.A.R. designed all other experiments, M.B.H., A.C.D. and T.A.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 and Supplementary References. (PDF 828 kb)
Rights and permissions
About this article
Cite this article
Hoppa, M., Lana, B., Margas, W. et al. α2δ expression sets presynaptic calcium channel abundance and release probability. Nature 486, 122–125 (2012). https://doi.org/10.1038/nature11033
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11033
This article is cited by
-
The circadian clock in the piriform cortex intrinsically tunes daily changes of odor-evoked neural activity
Communications Biology (2023)
-
Glutamate indicators with improved activation kinetics and localization for imaging synaptic transmission
Nature Methods (2023)
-
Cacna2d2 inhibits axonal regeneration following surgical decompression in a rat model of cervical spondylotic myelopathy
BMC Neuroscience (2022)
-
Pregabalin for chemotherapy-induced neuropathy: background and rationale for further study
Supportive Care in Cancer (2022)
-
A genome-wide association study of suicide attempts in the million veterans program identifies evidence of pan-ancestry and ancestry-specific risk loci
Molecular Psychiatry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.