Abstract
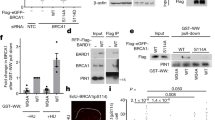
The breast cancer specific tumour suppressor protein, BRCA1 (refs 1,2), activates transcription when linked with a DNA-binding domain3,4 and is a component of the RNA polymerase II (Pol II) holoenzyme5,6. We show here that RNA helicase A (RHA) protein7,8 links BRCA1 to the holoenzyme complex. The region of BRCA1 which interacts with RHA and, thus, the holoenzyme complex, corresponds to subregions of the BRCT domain of BRCA1 ( ref. 9). This interaction was shown to occur in yeast nuclei, and expression in human cells of a truncated RHA molecule which retains binding to BRCA1 inhibited transcriptional activation mediated by the BRCA1 carboxy terminus. These data are the first to identify a specific protein interaction with the BRCA1 C-terminal domain and are consistent with the model that BRCA1 functions as a transcriptional coactivator.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1 . Science 266, 66–71 (1994).
Futreal, P.A. et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science 266, 120–122 ( 1994).
Monteiro, A.N., August, A. & Hanafusa, H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc. Natl Acad. Sci. USA 93, 13595–13599 (1996).
Chapman, M.S. & Verma, I.M. Transcriptional activation by BRCA1 . Nature 382, 678–679 (1996).
Scully, R. et al. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc. Natl Acad. Sci. U.S.A. 94, 5605–5610 (1997).
Neish, A.S., Anderson, S.F., Schlegel, B.P., Wei, W. & Parvin, J.D. Factors associated with the mammalian RNA polymerase II holoenzyme. Nucleic Acids Res. 26, 847– 853 (1998).
Lee, C.G. & Hurwitz, J. Human RNA helicase A is homologous to the maleless protein of Drosophila. J. Biol. Chem. 268, 16822–16830 (1993).
Zhang, S. & Grosse, F. Domain structure of human nuclear DNA helicase II (RNA helicase A). J. Biol. Chem. 272 , 11487–11494 (1997).
Koonin, E.V., Altschul, S.F. & Bork, P. BRCA1 protein products...Functional motifs. Nature Genet. 13, 266–268 (1996).
Nakajima, T., Uchida, C., Anderson, S.F., Parvin, J.D. & Montminy, M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 11, 738–747 ( 1997).
Bone, J.R. et al. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 8, 96– 104 (1994).
Nakajima, T. et al. RNA helicase A mediates association of CBP with RNA polymerase II. Cell 90, 1107–1112 ( 1997).
Szabo, C.I. & King, M.C. Inherited breast and ovarian cancer . Hum. Mol. Genet. 4, 1811– 1817 (1995).
Aspenstrom, P. & Olson, M.F. Yeast two-hybrid system to detect protein-protein interactions with Rho GTPases. Methods Enzymol. 256, 228–241 ( 1995).
Somasundaram, K. et al. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature 389, 187– 190 (1997).
Ouchi, T., Monteiro, A.N.A., August, A., Aaronson, S.A. & Hanafusa, H. BRCA1 regulates p53-dependent gene expression. Proc. Natl Acad. Sci. USA 95, 2302– 2306 (1998).
Tassan, J.P., Jaquenoud, M., Leopold, P., Schultz, S.J. & Nigg, E.A. Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C. Proc. Natl Acad. Sci. USA 92, 8871–8875 ( 1995).
Acknowledgements
We thank A. Monteiro and H. Hanafusa for the kind gift of GBD-BRCA1 constructs, and we thank M. Montminy, A. Dutta and D. Haile for helpful advice during the course of these experiments. This work was supported in part by NIH grant GM53504, a Junior Faculty Research Award from the American Cancer Society and also a Massachusetts Dept. of Public Health Breast Cancer Research Program grant to J.D.P. S.F.A. is supported by NRSA GM18829 from the NIH, and T.N. is supported by the Uehara Memorial Foundation and by the Otsuka Pharmaceutical Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, S., Schlegel, B., Nakajima, T. et al. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat Genet 19, 254–256 (1998). https://doi.org/10.1038/930
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/930