Abstract
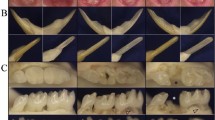
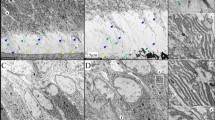
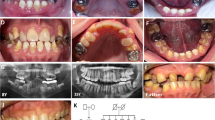
Dentinogenesis imperfecta 1 (DGI1, MIM 125490) is an autosomal dominant dental disease characterized by abnormal dentin production and mineralization. The DGI1 locus was recently refined to a 2-Mb interval on 4q21 (ref. 1). Here we study three Chinese families carrying DGI1. We find that the affected individuals of two families also presented with progressive sensorineural high-frequency hearing loss (gene DFNA39). We identified three disease-specific mutations within the dentin sialophosphoprotein gene (DSPP) in these three families. We detected a G→A transition at the donor-splicing site of intron 3 in one family without DFNA39, a mutation predicted to result in the skipping of exon 3. In two other families affected with both DGI1 and DFNA39, however, we identified two independent nucleotide transversions in exons 2 and 3 of DSPP, respectively, that cause missense mutations of two adjacent amino-acid residues in the predicted transmembrane region of the protein. Moreover, transcripts of DSPP previously reported to be expressed specifically in teeth2 are also detected in the inner ear of mice. We have thus demonstrated for the first time that distinct mutations in DSPP are responsible for the clinical manifestations of DGI1 with or without DFNA39.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aplin, H.M., Hirst, K.L. & Dixon, M.J. Refinement of the dentinogenesis imperfecta type II locus to an interval of less than 2 centiMorgans at chromosome 4q21 and the creation of a yeast artificial chromosome contig of the critical region. J. Dent. Res. 78, 1270–1276 (1999).
Ritchie, H.H., Hou, H., Veis, A. & Butler, W.T. Cloning and sequence determination of rat dentin sialoprotein, a novel dentin protein. J. Biol. Chem. 269, 3698–3702 (1994).
MacDougall, M. Refined mapping of the human dentin sialophosphoprotein (DSPP) gene within the critical dentinogenesis imperfecta type II and dentin dysplasia type II loci. Eur. J. Oral Sci. 106 (suppl. 1), 227–233 (1998).
Rowe, P.S. et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics 67, 54–68 (2000).
Hirst, K.L. et al. Elucidation of the sequence and the genomic organization of the human dentin matrix acidic phosphoprotein 1 (DMP1) gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics 42, 38–45 (1997).
Crosby, A.H., Edwards, S.J., Murray, J.C. & Dixon, M.J. Genomic organization of the human osteopontin gene: exclusion of the locus from a causative role in the pathogenesis of dentinogenesis imperfecta type II. Genomics 27, 155–160 (1995).
Crosby, A.H. et al. Mapping of the human and mouse bone sialoprotein and osteopontin loci. Mamm. Genome 7, 149–151 (1996).
Ritchie, H.H. et al. Dentin sialoprotein (DSP) transcripts: developmentally-sustained expression in odontoblasts and transient expression in pre-ameloblasts. Eur. J. Oral Sci. 105 (5 Pt 1), 405–413 (1997).
MacDougall, M. et al. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J. Biol. Chem. 272, 835–842 (1997).
Butler, W.T. Dentin matrix proteins. Eur. J. Oral Sci. 106 (suppl. 1), 204–210 (1998).
Takagi, Y. & Sasaki, S. A probable common disturbance in the early stage of odontoblast differentiation in Dentinogenesis imperfecta type I and type II. J. Oral Pathol. 17, 208–212 (1988).
Xia, J.H. et al. Mutations in the gene encoding gap junction protein β-3 associated with autosomal dominant hearing impairment. Nature Genet. 20, 370–373 (1998).
Richard, G. et al. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nature Genet. 20, 366–369 (1998).
Butler, W.T. Dentin matrix proteins and dentinogenesis. Connect. Tissue Res. 33, 59–65 (1995).
Bonadio, J. et al. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 87, 7145–7149 (1990).
Superti-Furga, A., Pistone, F., Romano, C. & Steinmann, B. Clinical variability of osteogenesis imperfecta linked to COL1A2 and associated with a structural defect in the type I collagen molecule. J. Med. Genet. 26, 358–362 (1989).
Nicholls, A.C., Oliver, J., McCarron, S., Winter, G.B. & Pope, F.M. Splice site mutation causing deletion of exon 21 sequences from the pro-α-2(I) chain of type I collagen in a patient with severe dentinogenesis imperfecta but very mild osteogenesis imperfecta. Hum. Mutat. 7, 219–227 (1996).
Fujisawa, R. & Kuboki, Y. Affinity of bone sialoprotein and several other bone and dentin acidic proteins to collagen fibrils. Calcif. Tissue Int. 51, 438–442 (1992).
Xiao, S. et al. Refinement of the locus for autosomal dominant hereditary gingival fibromatosis (GINGF) to a 3.8-cM region on 2p21. Genomics 68, 247–252 (2000).
Cottingham, R.W., Jr., Idury, R.M. & Schaffer, A.A. Faster sequential genetic linkage computations. Am. J. Hum. Genet. 53, 252–263 (1993).
Sobel, E. & Lange, K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am. J. Hum. Genet. 58, 1323–1337 (1996).
Acknowledgements
We thank the members of the three families for participation; W.R. Jin for DNA sequencing; H.W. Li and Y.Z. Shen for preparation of the mouse cochlear RNA; Y. Shen for discussions; F. Francis for critical reading; W. Chen for help in manuscript preparation; and Y. Li for technique assistance. This work was supported by the Life Science Special Fund for Human Genome Research granted by CAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, S., Yu, C., Chou, X. et al. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet 27, 201–204 (2001). https://doi.org/10.1038/84848
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84848
This article is cited by
-
Isolated dentinogenesis imperfecta: Novel DSPP variants and insights on genetic counselling
Clinical Oral Investigations (2024)
-
Identification of DSPP novel variants and phenotype analysis in dentinogenesis dysplasia Shields type II patients
Clinical Oral Investigations (2023)
-
Effects of Sr2+, BO33−, and SiO32− on Differentiation of Human Dental Pulp Stem Cells into Odontoblast-Like Cells
Biological Trace Element Research (2023)
-
Molecular Evolution of Tooth-Related Genes Provides New Insights into Dietary Adaptations of Mammals
Journal of Molecular Evolution (2021)
-
Compromised alveolar bone cells in a patient with dentinogenesis imperfecta caused by DSPP mutation
Clinical Oral Investigations (2019)