Abstract
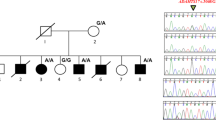
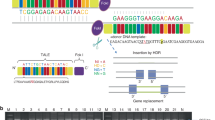
The autosomal recessive form of Robinow syndrome (RRS; MIM 268310) is a severe skeletal dysplasia with generalized limb bone shortening, segmental defects of the spine, brachydactyly and a dysmorphic facial appearance1,2,3. We previously mapped the gene mutated in RRS to chromosome 9q22 (ref. 4), a region that overlaps the locus for autosomal dominant brachydactyly type B (refs 5,6). The recent identification of ROR2, encoding an orphan receptor tyrosine kinase, as the gene mutated in brachydactyly type B (BDB1; ref. 7) and the mesomelic dwarfing in mice homozygous for a lacZ and/or a neo insertion into Ror2 (refs 8,9) made this gene a candidate for RRS. Here we report homozygous missense mutations in both intracellular and extracellular domains of ROR2 in affected individuals from 3 unrelated consanguineous families, and a nonsense mutation that removes the tyrosine kinase domain and all subsequent 3′ regions of the gene in 14 patients from 7 families from Oman. The nature of these mutations suggests that RRS is caused by loss of ROR2 activity. The identification of mutations in three distinct domains (containing Frizzled-like, kringle and tyrosine kinase motifs) indicates that these are all essential for ROR2 function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Teebi, A.S. Autosomal recessive Robinow syndrome. Am. J. Med. Genet. 35, 64–68 (1990).
Soliman, A.T, Rajab, A., Alsalmi, I. & Aziz Bedair, S.M. Recessive Robinow syndrome: with emphasis on endocrine functions. Metabolism 47, 1337–1343 (1998).
Wadia, R.S. Recessively inherited costovertebral segmentation defect with mesomelia and peculiar facies (Covesdem syndrome): a new genetic entity? J. Med. Genet. 15, 123–127 (1978).
Afzal, A.R. et al. Linkage of recessive Robinow syndrome to a 4 cM interval on chromosome 9q22. Hum. Genet. 106, 351–354 (2000).
Gong, Y. et al. Brachydactyly type B: clinical description, genetic mapping to chromosome 9q and evidence for a shared ancestral mutation. Am. J. Hum. Genet. 64, 570–577 (1999).
Oldridge, M. et al. Brachydactyly type B: linkage to chromosome 9q22 and evidence for genetic heterogeneity. Am. J. Hum. Genet. 64, 578–583 (1999).
Oldridge, M. et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nature Genet. 24, 275–278 (2000).
De Chiara, T.M. et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nature Genet. 24, 271–274 (2000).
Takeuchi, S. et al. Mouse ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 5, 71–78 (2000).
Aksit, S. et al. Is the frequency of Robinow syndrome relatively high in Turkey? Four more case reports. Clin. Genet. 52, 226–230 (1997).
Masiakowski, P. & Carroll, R.D. A novel family of cell surface receptors with tyrosine kinase-like domain. J. Biol. Chem. 267, 26181–26190 (1992).
Wilson, C., Goberdhan, D.C. & Steller, H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor tyrosine kinases. Proc. Natl Acad. Sci. USA 90, 7109–7113 (1993).
Oishi, I. et al. A novel Drosophila receptor tyrosine kinase expressed specifically in the nervous system. J. Biol. Chem. 272, 11916–11923 (1997).
Forrester, W.C., Dell, M., Perens, E. & Garriga, G. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature 400, 881–885 (1999).
Frischmeyer, P.A. & Dietz, H.C. Nonsense mediated mRNA decay in health and disease. Hum. Mol. Genet. 8, 1893–1900 (1999).
Vihinen, M. et al. Structural basis for X-linked agammaglobulinemia. A tyrosine kinase disease. Proc. Natl Acad. Sci. USA 91, 12803–12807 (1994).
Vorechovsky, I. et al. DNA-based mutation analysis of Bruton's tyrosine kinase gene in patients with X-linked agammaglobulinemia. Hum. Mol. Genet. 4, 51–58 (1995).
Xu, Y.K. & Nusse, R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr. Biol. 4, R405–R406 (1998).
Masiakowski, P. & Yancopoulos, G.D. The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Curr. Biol. 4, R407 (1998).
Robertson, S.C. et al. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulphide bond in the third immunoglobulin-like domain. Proc. Natl Acad. Sci. USA 95, 4567–4572 (1998).
Gherardi, E., Gonzalez Manzano, R., Cottage, A., Hawker, K. & Aparicio, S. Evolution of plasminogen-related growth factors (HGF/SF and HGF1/MSP). Ciba Found. Symp. 212, 24–41 (1997).
Castellino, F.J. & McCance, S.G. The kringle domains of human plasminogen. Ciba Found. Symp. 212, 46–65 (1997).
Schuster, V. et al. Homozygous mutations in the plasminogen gene of two unrelated girls with ligneous conjunctivitis. Blood 90, 958–966 (1997).
Schuster, V. et al. Compound-heterozygous mutations in the plasminogen gene predispose to the development of ligneous conjunctivitis. Blood 93, 3457–3466 (1999).
Taylor, S.I. et al. Genetic basis of endocrine disease 1. Molecular genetics of insulin resistant diabetes mellitus. J. Clin. Endocrinol. Metab. 73, 1158–1163 (1991).
Polinkovsky, A. et al. Mutations in CDMP1 cause autosomal dominant brachydactyly type C. Nature Genet. 17, 18–19 (1997).
Thomas, J.T. et al. A human chondrodysplasia due to a mutation in a TGFb superfamily member. Nature Genet. 12, 315–317 (1996).
Thomas, J.T. et al. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1. Nature Genet. 17, 58–64 (1997).
Robinow, M. The Robinow (fetal face) syndrome: a continuing puzzle. Clin. Dysmorphol. 2, 189–198 (1993).
Kantaputra, P.N., Gorlin, R.J., Ukarapol, N., Unachak, K. & Sudasna, J. Robinow (fetal face) syndrome: report of a boy with dominant type and an infant with recessive type. Am. J. Med. Genet. 84, 1–7 (1999).
Acknowledgements
We thank S. Mansour for suggestions; the Birth Defects Foundation (UK) for support; and The Wandsworth Heart & Stroke Study (F.P. Cappuccio and D.G. Cook) for the DNA samples from individuals of Pakistani descent. A.R.A., M.O. and A.O.M.W. were supported by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Afzal, A., Rajab, A., Fenske, C. et al. Recessive Robinow syndrome, allelic to dominant brachydactyly type B, is caused by mutation of ROR2. Nat Genet 25, 419–422 (2000). https://doi.org/10.1038/78107
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/78107
This article is cited by
-
Role of the Ror family receptors in Wnt5a signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Genomic frontiers in congenital heart disease
Nature Reviews Cardiology (2022)
-
Planar cell polarity pathway in kidney development, function and disease
Nature Reviews Nephrology (2021)
-
Disorders of FZ-CRD; insights towards FZ-CRD folding and therapeutic landscape
Molecular Medicine (2020)
-
ROR2 induces cell apoptosis via activating IRE1α/JNK/CHOP pathway in high-grade serous ovarian carcinoma in vitro and in vivo
Journal of Translational Medicine (2019)