Abstract
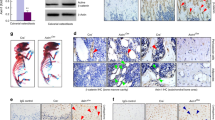
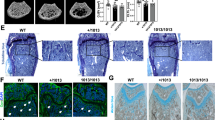
The c-Abl protein is a non-receptor tyrosine kinase involved in many aspects of mammalian development. c-Abl kinase is widely expressed, but high levels are found in hyaline cartilage in the adult, bone tissue in newborn mice, and osteoblasts and associated neovasculature at sites of endochondrial ossification in the fetus1,2. Mice homozygous for mutations in the gene encoding c-Abl (Abl) display increased perinatal mortality, reduced fertility, foreshortened crania and defects in the maturation of B cells in bone marrow3,4. Here we demonstrate that Abl−/− mice are also osteoporotic. The long bones of mutant mice contain thinner cortical bone and reduced trabecular bone volume. The osteoporotic phenotype is not due to accelerated bone turnover—both the number and activity of osteoclasts are similar to those of control littermates—but rather to dysfunctional osteoblasts. In addition, the rate of mineral apposition in the mutant animals is reduced. Osteoblasts from both stromal and calvarial explants showed delayed maturation in vitro as measured by expression of alkaline phosphatase (ALP), induction of mRNA encoding osteocalcin and mineral deposition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
O'Neill, A.J., Cotter, T.G., Russell, J.M. & Gaffney, E.F. Abl expression in human fetal and adult tissues, tumours, and tumour microvessels . J. Pathol. 183, 325–329 (1997).
Muller, R., Slamon, D.J., Trembley, J.M., Cline, M.J. & Verma, I.M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature 299, 640–644 ( 1982).
Schwartzberg, P.L. et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65, 1165–1175 ( 1991).
Tybulewicz, V.L.J., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 ( 1991).
Tondravi, M.M. et al. Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81– 84 (1997).
Blair, H.C., Kahn, A.J., Crouch, E.C., Jeffrey, J.J. & Teitelbaum, S.L. Isolated osteoclasts resorb the organic and inorganic components of bone. J. Cell Biol. 102, 1164 –1172 (1986).
Owen, M. & Friedenstein, A.J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found. Symp. 136, 42–60 (1988).
Stein, G.S., Lian, J.B. & Owen, T.A. Relationships of cell growth to the regulation of tissue specific gene expression during osteoblast differentiation. FASEB J. 4, 3111–3123 ( 1990).
Cook, H.C. Manual of Histological Demonstration Techniques (Butterworth, London, 1974).
Aubin, J.E., Liu, F., Malaval, L. & Gupta, A.K. Osteoblast and chondroblast differentiation. Bone 17, 77S –83S (1995).
Sawyers, C.L., McLaughlin, J., Goga, A., Havlik, M. & Witte, O.N. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell 77, 121– 131 (1994).
Wen, S.T., Jackson, P.K. & Van Etten, R.A. The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 15, 1583– 1595 (1996).
Quarles, L.D., Yohay, D.A., Lever, L.W., Caton, R. & Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development . J. Bone Miner. Res. 7, 683– 692 (1992).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45– 51 (1997).
Ducy, P., Zhang, R., Geoffroy, V., Ridall, A.L. & Karsenty, G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747– 754 (1997).
Mundlos, S. et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89, 773– 779 (1997).
Otto, F. et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 ( 1997).
Moursi, A.M. et al. Fibronectin regulates calvarial osteoblast differentiation J. Cell Sci. 109, 1369– 1380 (1996).
Lewis, J.M., Baskaran, R., Taagepera, S., Schwartz, M.A. & Wang, J.Y. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc. Natl Acad. Sci. USA 93, 15174–15179 (1996).
Hardin, J.D. et al. Bone marrow B lymphocyte development in c-abl deficient mice . Cell. Immunol. 165, 44– 54 (1995).
Takahashi, N. et al. Induction of calcitonin receptors by 1 a, 25-dihydroxyvitamin D3 in osteoclast-like multinucleated cells formed from mouse bone marrow cells . Endocrinology 123, 1504– 1510 (1988).
Engleman, V.W. et al. A peptidomimetic antagonist of the α(v)β3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J. Clin. Invest. 99, 2284–2292 (1997).
Acknowledgements
We thank P. Schwartzberg, P. Ducy, G. Karsenty, M. Sahni and R. Majeska for helpful discussions and technical advice, and Genetics Institute for BMPs 2 and 4. S.L.T was supported by grants DE05413 and AR32788 from Shriner's Hospital for Crippled Children. M.M.T. was supported by a grant AR44089 from NIH. B.L. is an associate, S.B. a research assistant and S.P.G. an investigator of Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, B., Boast, S., de los Santos, K. et al. Mice deficient in Abl are osteoporotic and have defects in osteoblast maturation. Nat Genet 24, 304–308 (2000). https://doi.org/10.1038/73542
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/73542
This article is cited by
-
Abelson kinase’s intrinsically disordered region plays essential roles in protein function and protein stability
Cell Communication and Signaling (2021)
-
Tankyrase inhibitor XAV-939 enhances osteoblastogenesis and mineralization of human skeletal (mesenchymal) stem cells
Scientific Reports (2020)
-
Osteogenic differentiation of skeletal muscle progenitor cells is activated by the DNA damage response
Scientific Reports (2019)
-
c-Abl regulates gastrointestinal muscularis propria homeostasis via ERKs
Scientific Reports (2017)
-
Germline mutations in ABL1 cause an autosomal dominant syndrome characterized by congenital heart defects and skeletal malformations
Nature Genetics (2017)