Abstract
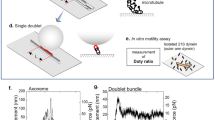
Eukaryotic flagella beat rhythmically1. Dynein is a protein that powers flagellar motion, and oscillation may be inherent to this protein2,3,4,5. Here we determine whether oscillation is a property of dynein arms themselves or whether oscillation requires an intact axoneme6, which is the central core of the flagellum and consists ofa regular array of microtubules. Using optical trapping nanometry7,8, we measured the force generated by a few dynein arms on an isolated doublet microtubule. When the dynein arms on the doublet microtubule contact a singlet microtubule and are activated by photolysis of caged ATP8, they generate a peak force of ∼6 pN and move the singlet microtubule over the doublet microtubule in a processive manner. The force and displacement oscillate with a peak-to-peak force and amplitude of ∼2 pN and ∼30 nm, respectively. The geometry of the interaction indicates that very few (possibly one) dynein arms are needed to generate the oscillation. The maximum frequency of the oscillation at 0.75 mM ATP is ∼70 Hz; this frequency decreases as the ATP concentration decreases. A similar oscillatory force is also generated by inner dynein arms alone on doublet microtubules that are depleted of outer dynein arms. The oscillation of the dynein arm may be a basic mechanism underlying flagellar beating.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brokaw, C. J. Operation and regulation of the flagellar oscillator. Cell Movement 1, 267–279 (1989).
Shingyoji, C., Murakami, A., & Takahashi, K. Local reactivation of Triton-extracted flagella by iontophoretic application of ATP. Nature 265, 269–270 (1977).
Takahashi, K., Shingyoji, C., & Kamimura, S. Microtubule sliding in reactivated flagella. Soc. Exp. Biol. Symp. 35, 159–177 (1982).
Shingyoji, C. & Takahashi, K. Cyclical bending movements induced locally by successive iontophoretic application of ATP to an elastase-treated flagellar axoneme. J. Cell Sci. 108, 1359–1369 (1995).
Kamimura, S. & Kamiya, R. High-frequency nanometre-scale vibration in ‘quiescent’ flagellar axonemes. Nature 340, 476–478 (1989).
Smith, E. F. & Sale, W. S. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science 257, 1557–1559 (1992).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Higuchi, H., Muto, E., Inoue, Y. & Yanagida, T. Kinetics of force generation by single kinesin molecules activated by laser photolysis of caged ATP. Proc. Natl Acad. Sci. USA 94, 4359–4400 (1997).
Gibbons, I. R. Dynein ATPases as microtubule motors. J. Biol. Chem. 263, 15837–15840 (1988).
Sale, W. S. & Satir, P. Direction of active sliding of microtubules in Tetrahymena cilia. Proc. Natl Acad. Sci. USA 74, 2045–2059 (177).
Fox, L. A. & Sale, W. S. Direction of force generated by the inner row of dynein arms on flagellar microtubules. J. Cell Biol. 105, 1781–1787 (1987).
Vale, R. D. & Toyoshima, Y. Y. Rotation and translocation of microtubules in vitro induced by dyneins from Tetrahymena cilia. Cell 52, 459–469 (1988).
Sale, W. S. & Fox, L. A. Isolated β-heavy chain subunit of dynein translocates microtubules in vitro. J. Cell Biol. 107, 1793–1797 (1988).
Yokota, E. & Mabuchi, I. C/A dynein isolated from sea urchin sperm flagellar axonemes. Enzymatic properties and interaction with microtubules. J. Cell Sci. 107, 353–361 (1994).
Johnson, K. A. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu. Rev. Biophys. Chem. 14, 161–188 (1985).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Ishijima, A. et al. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem. Biophys. Res. Commun. 199, 1057–1063 (1994).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Goodenough, U. & Heuser, J. Structural comparison of purified dynein proteins with in situ dynein arms. J. Mol. Biol. 180, 1083–1118 (1984).
Sale, W. S., Goodenough, U. W. & Heuser, J. E. The substructure of isolated and in situ outer dynein arms of sea urchin sperm flagella. J. Cell Biol. 101, 1400–1412 (1985).
Wang, Z., Khan, S. & Sheetz, M. Single cytoplasmic dynein molecule movements: characterization and comparison with kinesin. Biophys. J. 69, 2011–2023 (1995).
Thomas, N. & Thornhill, R. A. The physics of biological molecular motors. J. Phys. D 31, 253–266 (1998).
Thomas, N. & Thornhill, R. A. Possible origin of tension oscillations in muscle preparations. J. Physiol. (Lond.) 491, 124P (1996).
Moss, A. G., Sale, W. S., Fox, L. A. & Witman, G. B. The α subunit of sea urchin sperm outer arm dynein mediates structural and rigor binding to microtubules. J. Cell Biol. 118, 1189–1200 (1992).
Moss, A. G., Gatti, J.-L. & Witman, G. B. The motile β/IC1 subunit of sea urchin sperm outer arm dynein does not form a rigor bond. J. Cell Biol. 118, 1177–1188 (1992).
Omoto, C. K. Mechanochemical coupling in eukaryotic flagella. J. Theor. Biol. 137, 163–169 (1989).
Shimizu, T., Marchese-Ragona, S. P., & Johnson, K. A. Activation of the dynein adenosinetriphosphatase by cross-linking to microtubules. Biochemistry 28, 7016–7021 (1989).
Gee, M. A., Heuser, J. A. & Vallee, R. B. An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636–639 (1997).
Gibbons, B. H. & Gibbons, I. R. The effect of partial extraction of dynein arms on the movement of reactivated sea-urchin sperm. J. Cell Sci. 13, 337–357 (1973).
Katayama, E. The effects of various nucleotides on the structure of actin-attached myosin subfragment-1 studied by quick-freeze deep-etch electron microscopy. J. Biochem. 106, 751–770 (1989).
Acknowledgements
We thank K. Takahashi and R. A. Thornhill for discussion and critical reading of the manuscript, and E. Muto and Y. Inoue for suggestions about the preparation of microtubules. This work was supported by a Kurata Research Grant and a Grant from Nissan Science Foundation (to C.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shingyoji, C., Higuchi, H., Yoshimura, M. et al. Dynein arms are oscillating force generators. Nature 393, 711–714 (1998). https://doi.org/10.1038/31520
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/31520
This article is cited by
-
Versatile properties of dynein molecules underlying regulation in flagellar oscillation
Scientific Reports (2023)
-
The multiscale physics of cilia and flagella
Nature Reviews Physics (2020)
-
Dynein harnesses active fluctuations of microtubules for faster movement
Nature Physics (2020)
-
C-terminal Tail of β-Tubulin and its Role in the Alterations of Dynein Binding Mode
Cell Biochemistry and Biophysics (2020)
-
Human sperm steer with second harmonics of the flagellar beat
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.