Abstract
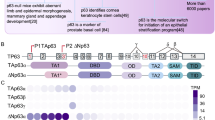
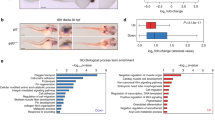
The p63 gene, a homologue of the tumour-suppressor p53 (1–5), is highly expressed in the basal or progenitor layers of many epithelial tissues1. Here we report that mice homozygous for a disrupted p63 gene have major defects in their limb, craniofacial and epithelial development. p63 is expressed in the ectodermal surfaces of the limb buds, branchial arches and epidermal appendages, which are all sites of reciprocal signalling that direct morphogenetic patterning of the underlying mesoderm. The limb truncations are due to a failure to maintain the apical ectodermal ridge, a stratified epithelium, essential for limb development. The embryonic epidermis of p63 −/− mice undergoes an unusual process of non-regenerative differentiation, culminating in a striking absence of all squamous epithelia and their derivatives, including mammary, lacrymal and salivary glands. Taken together, our results indicate that p63 is critical for maintaining the progenitor-cell populations that are necessary to sustain epithelial development and morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yang, A. et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998).
Augustin, M., Bamberger, C., Paul, D. & Schmale, H. Cloning and chromosomal mapping of the human p53-related KET gene to chromosome 3q27 and its murine homolog Ket to mouse chromosome 16. Mamm. Genome 11, 899–902 (1998).
Osada, M. et al. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nature Med. 4, 839–843 (1998).
Trink, B. et al. Anew human p53 homologue. Nature Med. 4, 747–748 (1998).
Ko, L. J. & Prives, C. p53: puzzle and paradigm. Genes Dev. 10, 1054–1072 (1996).
Kaghad, M. et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell 90, 809–819 (1997).
Mansour, S. L., Thomas, K. R. & Capecchi, M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336, 348–352 (1988).
Tickle, C. Vertebrate limb development. Curr. Opin. Genet. Dev. 5, 478–484 (1995).
Johnson, R. L. & Tabin, C. J. Molecular models for vertebrate limb development. Cell 90, 979–990 (1997).
Kelley, R. O. & Fallon, J. F. Ultrastructural analysis of the apical ectodermal ridge during vertebrate limb morphogenesis. I. The human forelimb with special reference to gap junctions. Dev. Biol. 51, 241–256 (1976).
Francis-West, P., Ladher, R., Barlow, A. & Graveson, A. Signalling interactions during facial development. Mech. Dev. 75, 3–28 (1998).
Crossley, P. H., Minowada, G., MacArthur, C. A. & Martin, G. R. Roles for FGF-8 in the induction, initiation, and maintenance of chick limb development. Cell 84, 127–136 (1996).
Vogel, A., Rodriguez, C. & Izpisua-Belamonte, J. C. Involvement of FGF-8 in initiation, outgrowth, and patterning of the vertebrate limb. Development 122, 1737–1750 (1996).
Loomis, C. A., Kimmel, R. A., Tong, C. X., Michaud, J. & Joyner, A. L. Analysis of the genetic pathway leading to formation of ectopic apical ectodermal ridges in mouse Engrailed-1 mutant limbs. Development 125, 1137–1148 (1998).
Roberts, B., Lyons, G., Simandl, B. K., Kuroiwa, A. & Buckingham, M. The apical ectodermal ridge regulates Hox-7 and Hox-8 gene expression in developing chick limb buds. Genes Dev. 5, 2362–2374 (1991).
Wang, Y. & Sassoon, D. Ectoderm–mesenchyme and mesenchyme–mesenchyme interactions regulate Msx-1 expression and cellular differentiation in the murine limb bud. Dev. Biol. 168, 374–382 (1995).
Chen, H. et al. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nature Genet. 19, 51–55 (1998).
Riddle, R. D. et al. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell 83, 631–640 (1995).
Parr, B. A. & McMahon, A. P. Dorsalizing signal Wnt-7a required for normal polarity of D–V and A–P axes of mouse limb. Nature 374, 350–353 (1995).
Pellegrini, G. et al. Location and clonal analysis of stem cells and their differentiated progeny in the human occular surface. J. Cell Biol.(in the press).
Byrne, C., Tainsky, M. & Fuchs, E. Programming gene expression in developing epidermis. Development 120, 2369–2383 (1994).
Rheinwald, J. G. & Green, H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–344 (1975).
Barrandon, Y. & Green, H. Three clonal types of keratinocytes with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306 (1987).
Barrandon, Y. The epidermal stem cell: an overview. Dev. Biol. 4, 209–215 (1993).
Watt, F. M. Epidermal stem cells: markers, patterning and the control of stem cell fate. Phil. Trans. R. Soc. Lond. B 358, 553–560 (1991).
Greer, J. & Roop, D. Loricrin, a major keratinocyte envelope protein, is expressed late in development. J. Invest. Term. 96, 553–560 (1991).
Calautti, E., Missero, C., Stein, P. L., Ezzell, R. M. & Dotto, G. P. Fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Dev. 9, 2279–2291 (1995).
Jan, Y. N. & Jan, L. Y. Asymmetric cell division. Nature 392, 775–778 (1998).
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Jacks, T. et al. Tumor spectrum analysis in p53 mutant mice. Curr. Biol. 4, 1–7 (1994).
Acknowledgements
We thank H. Green, M. Kirschner, P. Dotto, B. Olsen, N. Fukai, T. Rapoport, B.Quade, J. Glickman, P. Ferrara and T. Bestor for their support and for discussion; H. Green, G. Martin, A. McMahon, R. Johnson, A. Joyner and P. Dotto for reagents; L. Du for blastocyst injections; H. Liu for the 129 mouse genomic library; and J. Williams for histology preparations. This work was supported by grants from the NIH to R.B., C.T., A.S. and F.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, A., Schweitzer, R., Sun, D. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999). https://doi.org/10.1038/19539
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/19539
This article is cited by
-
The urothelial gene regulatory network: understanding biology to improve bladder cancer management
Oncogene (2024)
-
The long non-coding RNA NEAT1 is a ΔNp63 target gene modulating epidermal differentiation
Nature Communications (2023)
-
p63: a crucial player in epithelial stemness regulation
Oncogene (2023)
-
Disease-related p63 DBD mutations impair DNA binding by distinct mechanisms and varying degree
Cell Death & Disease (2023)
-
DARPins detect the formation of hetero-tetramers of p63 and p73 in epithelial tissues and in squamous cell carcinoma
Cell Death & Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.