Abstract
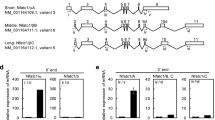
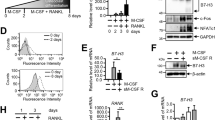
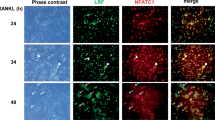
The tumour-necrosis-factor-family molecule osteoprotegerin ligand (OPGL; also known as TRANCE, RANKL and ODF) has been identified as a potential osteoclast differentiation factor and regulator of interactions between T cells and dendritic cells in vitro. Mice with a disrupted opgl gene show severe osteopetrosis and a defect in tooth eruption, and completely lack osteoclasts as a result of an inability of osteoblasts to support osteoclastogenesis. Although dendritic cells appear normal, opgl-deficient mice exhibit defects in early differentiation of T and B lymphocytes. Surprisingly, opgl-deficient mice lack all lymph nodes but have normal splenic structure and Peyer's patches. Thus OPGL is a new regulator of lymph-node organogenesis and lymphocyte development and is an essential osteoclast differentiation factor in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Felix, R., Hofstetter, W. & Cecchini, M. G. Recent developments in the understanding of the pathophysiology of osteopetrosis. Eur. J. Endocrinol. 134, 143–156 (1996).
Roodman, G. D. Advances in bone biology: the osteoclast. Endocr. Hev. 17, 308–332 (1996).
Manolagas, S. C. & Jilka, R. L. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. New Engl. J. Med. 332, 305–311 (1995).
Popoff, S. N. & Schneider, G. B. Animal models of osteopetrosis: the impact of recent molecular developments on novel strategies for therapeutic intervention. Mol. Med. Today 2, 349–358 (1996).
Teitelbaum, S. I. The osteoclast and osteoporosis. Mt Sinai J. Med. 63, 399–402 (1996).
Reddi, A. H. Bone morphogenesis and modeling: soluble signals sculpt osteosomes in the solid state. Cell 89, 159–161 (1997).
Mostov, K. & Werb, Z. Journey across the osteoclast. Science 276, 219–220 (1997).
Rosen, CJ. & Kessenich, C. R. The pathophysiology and treatment of postmenopausal osteoporosis. An evidence-based approach to estrogen replacement therapy. Endocr. Metab. Clin. North Am. 26, 295–311 (1997).
Kanis, J. A. Bone and cancer: pathophysiology and treatment of metastases. Bone 17 (suppl.), 101–105 (1995).
Seitz, M. & Hunstein, W. Enhanced prostanoid release from monocytes of patients with rheumatoid arthritis and active systemic lupus erythematosus. Annu. Rheum. Dis. 44, 438–445 (1985).
Yoshida, H. et al. The murine mutation osteopetrosis in in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 (1990).
Wiktor-Jedrzejczak, W. et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl Acad. Sci. USA 87, 4828–4832 (1990).
Lagasse, E. & Weissman, I. L. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell 89, 1021–1031 (1997).
Begg, S. K. et al. Delayed hematopoietic development in osteopetrotic (op/op) mice. J. Exp. Med. 177, 237–242 (1993).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Anderson, D. M. et al. Ahomologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390, 175–179 (1997).
Wong, B. R. et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 272, 25190–25194 (1997).
Wong, B. R. et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J. Exp. Med. 186, 2075–2080 (1997).
Grigoriadis, A. E. et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 (1994).
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Iotsova, V. et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nature Med. 3, 1285–1289 (1997).
Franzoso, G. et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 11, 3482–3496 (1997).
Kurihara, N. et al. Generation of osteoclasts from isolated heatopoietic progenitor cells. Blood 74, 1295–1302 (1989).
Kurihara, N., Chenu, C., Miller, M., Civin, C. & Roodman, G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology 126, 2733–2741 (1990).
Anderson, G., Moore, N. C., Owen, J. J. & Jenkinson, E. J. Cellular interactions in thymocyte development. Annu. Rev. Immunol. 14, 73–99 (1996).
von Boehmer, H. & Fehling, H. J. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 15, 433–452 (1997).
Akagawa, K. S. et al. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood 88, 4029–4039 (1996).
Steinman, R. M., Kaplan, G., Witmer, M. D. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J. Exp. Med. 149, 1–16 (1979).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 (1992).
Saoulli, K. et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. med. 187, 1849–1862 (1998).
DeBenedette, M. A., Shahinian, A., Mak, T. W. & Watts, T. H. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 158, 551–559 (1997).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Koni, P. A. et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity 6, 491–500 (1997).
Alimzhanov, M. B. et al. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc. Natl Acad. Sci. USA 94, 9302–9307 (1997).
Matsumoto, M. et al. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 271, 1289–1291 (1996).
Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 (1996).
Futterer, A., Mink, K., Luz, A., Kosco-Vilbois, M. H. & Pfeffer, K. The lymphotoxin β receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9, 59–70 (1998).
Rennert, P. D., James, D., Mackey, F., Browning, J. L. & Hochman, P. S. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity 9, 71–79 (1998).
Bailey, R. P. & Weiss, L. Ontogeny of human fetal lymph nodes. Am. J. Anat. 142, 15–27 (1975).
Cheng, A. M. et al. The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc. Natl Acad. Sci. USA 94, 9797–9801 (1997).
Clements, J. L. et al. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281, 416–419 (1998).
Fehling, H. J., Krotkova, A., Saint-Ruf, C. & von Boehmer, H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature 375, 795–798 (1995).
Irving, B. A., Alt, F. W. & Killeen, N. Thymocyte development in the absence of pre-T cell receptor extracellular immunoglobulin domains. Science 280, 905–908 (1998).
Turner, M. et al. Arequirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7, 451–460 (1997).
Peschon, J. J. et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180, 1955–1960 (1994).
Maraskovsky, E. et al. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89, 1011–1019 (1997).
Mombaerts, P. et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75, 274–282 (1993).
Simonet, W. S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).
Takahashi, N. et al. Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology 128, 1792–1796 (1991).
Acknowledgements
We thank D. Bouchard for cell sorting; D. Duryea and C. Burgh for technical assistance; R. Boyd, M. Bachmann, G. Wick, and N.Romani for reagents; M. Saunders for editing this manuscript; and R. Yoshida, K. Bachmaier, A.Hakem, I.Kozieradzki, T. Sasaki and M. Nghiem for comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, YY., Yoshida, H., Sarosi, I. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999). https://doi.org/10.1038/16852
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/16852
This article is cited by
-
Oxyresveratrol attenuates bone resorption by inhibiting the mitogen-activated protein kinase pathway in ovariectomized rats
Nutrition & Metabolism (2024)
-
Non-biofilm-forming Staphylococcus epidermidis planktonic cell supernatant induces alterations in osteoblast biological function
Scientific Reports (2024)
-
Oral health outcomes in an HIV cohort with comorbidities- implementation roadmap for a longitudinal prospective observational study
BMC Oral Health (2023)
-
Small-molecule amines: a big role in the regulation of bone homeostasis
Bone Research (2023)
-
Mesenchymal stem cell-derived apoptotic bodies alleviate alveolar bone destruction by regulating osteoclast differentiation and function
International Journal of Oral Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.