Abstract
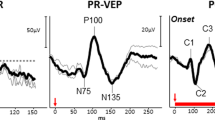
Pattern and flash visual evoked cortical potentials (PVEP, FVEP), and pattern electroretinograms, (PERG) were recorded in 13 affected individuals from 8 families with DOA. These were selected as representative from 87 affected members of 21 pedigrees with DOA who were examined, and who underwent genetic linkage analysis. Linkage to the OPA1 locus on chromosome 3q 28-qter was demonstrated in all families. VA ranged from 6/9 to HM; visual fields showed a variable centro-caecal defect; SLO (when performed) showed diffuse nerve fibre loss; MRI (when performed) showed small intra-orbital optic nerves. In 9/13 patients the PVEP was absent in one or both eyes. Most recordable PVEPs were of abnormal latency, but the delays were not marked (peak times 116–135 msec); amplitudes were low or subnormal. PERG fell within the normal range in 9 eyes of 7 patients. 14 eyes showed an abnormal N95:P50 ratio in keeping with ganglion cell dysfunction. Some severely affected eyes showed P50 component involvement, but in no eye was the PERG extinguished. Significant interocular asymmetries in at least one electrophysiological measure were present in 6/13 patients. Colour contrast thresholds were significantly elevated for all three colour confusion axes, with tritan being most affected.
Similar content being viewed by others
References
Kjer P. Infantile optic atrophy with dominant mode of inheritance: a clinical and genetic study of 19 Danish families. Acta Ophthalmol 1959; 37 (Suppl 54): 1–146.
Eliott MD. Visual prognosis in autosomal dominant optic atrophy (Kjer type). Am J Ophthalmol 1993; 115: 360–7.
Batten B. family suffering from hereditary optic atrophy. Trans Ophthalmol Soc UK 1896; 16: 125.
Caldwell JBH, Howard RO, Riggs LA. Dominant juvenile optic atrophy: A study of two families and review of the hereditary disease in childhood. Arch Ophthalmol 1971; 85: 133–47.
Smith DP. Diagnostic criteria in dominantly inherited juvenile optic atrophy: A report of three new families. Am J Opt & Physiol Optics 1972; 49: 183–200.
Hoyt CS. Autosomal dominant optic atrophy: a spectrum of disability. Ophthalmology 1980; 87: 245–51.
Jaeger W. Diagnosis of dominant infantile optic atrophy in early childhood. Ophthalmic Paediatrics and Genetics 1988; 9: 7–11.
Kline LB, Glaser JS. Dominant optic atrophy. The clinical profile. Arch Ophthalmol 1979; 97: 1680–6.
Johnston PB, Gaster RN, Smith VC, Tripathi RC. A clinicopathological study of autosomal dominant optic atrophy. Am J Ophthalmol 1979; 88: 868–75.
Kjer P. Histopathology of eye, optic nerve and brain in a case of dominant optic atrophy. Acta Ophthalmol 1982; 61: 300–12.
Eiberg H, Kjer B, Kjer P, Rosenberg T. Dominant optic atrophy (OPA1) mapped to chromosome 3q region. I. Linkage analysis. Hum Molec Genet 1994; 3: 977–80.
Bonneau D, Souied E, Gerber S, Rozet J-M, D'Haens E, Journel H, et al. No evidence of genetic heterogeneity in dominant optic atrophy. J Med Genet 1995; 32: 951–3.
Brown J, Fingert JH, Taylor CM, Lake M, Sheffield VC, Stone EM. Clinical and genetic analysis of a family affected with dominant optic atrophy (OPA1). Arch Ophthalmol 1997; 115: 95–9.
Johnston RL, Burdon MA, Spalton DJ, Bryant SP, Behnam JT, Seller MJ. Dominant op-tic atrophy, Kjer type: Linkage analysis and clinical features in a large British pedigree. Arch Ophthalmol 1997; 115: 100–3.
Jonasdottir A, Eiberg H, Kjer B, Kjer P, Rosenberg T. Refinement of the dominant optic atrophy locus (OPA1) to a 1.4-cM interval on chromosome 3q28-3q29, within a 3-Mb YAC contig. Hum Genet 1997; 99: 115–20.
Stoilova D, Child A, Desai SP, Sarfarazi M. Refinement of the locus for autosomal dominant juvenile optic atrophy to a 2cM region on 3q28. Ophthalmic Genetics 1997; 18: 1–6.
Votruba M, Moore AT, Bhattacharya SS. Genetic refinement of dominant optic atrophy (OPA1) locus to within a 2cM interval of chromosome 3q. J Med Genet 1997a; 34: 117–21.
Votruba M, Fitzke FW, Holder GE, Carter AC, Bhattacharya SS, Moore AT. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol 1997b; in press.
Fitzke FW, Poinoosawmy D, Ernst W, Hitchings RA. Peripheral displacement thresholds in normals, ocular hypertensives and glaucoma. In: Greve EL, Heijl A, ed. Seventh International Visual Field Symposium. 1987: 447–52.
Cioffi GA, Robin AL, Eastman RD, Perell HF, Sarfarazi FA, Kelman SE. Confocal laser scanning ophthalmoscope. Reproducibility of optic nerve head topographic measurements with the confocal laser scanning ophthalmoscope. Ophthalmology 1993; 100: 57–62.
Arden GB, Gunduz K, Perry, S. Colour vision testing with a computer graphics system. Clin Vis Sci 1988; 2: 303–20.
Berninger TA, Canning C, Strong N, Gunduz K, Arden GB. Using Argon laser blue light reduces Ophthalmologists' colour contrast sensitivity. Arch Ophthalmol 1989; 107: 1453–8.
Gunduz K, Arden GB. Changes in colour contrast sensitivity associated with operating Argon lasers. Br J Ophthalmol 1989; 73: 241–6.
Harding GFA, Odom JV, Spileers W, Spekreijse H. Standard for Visual Evoked Potentials 1995. Vision Res 1996; 36: 3567–72.
Marmor MF, Holder GE, Porciatti V, Trick G, Zrenner E. Guidelines for pattern electroretinography. Recommendations by the International Society For Clinical Electrophysiology of Vision. Doc Ophthalmol 1996; 91: 291–8.
Holder GE. Recording the pattern electroretinogram with the Arden gold foil electrode. J Electrophysiol Technol 1988; 14: 183–90.
Odom JV, Holder GE, Feghali JG, Cavender S. Pattern electroretinogram intrasession reliability: A two center comparison. Clin Vis Sci 1992; 7: 263–82.
Arden GB, Hogg CR, Holder GE. Gold foil electrodes: a two centre study of electrode reliability. Doc Ophthalmol 1994; 86: 275–84.
Harding GFA. The visual evoked response. in Roper-Hall MJ et al., eds. Advances in Ophthalmology. Karger, Basel, 1974, 2–28.
Holder GE. Pattern ERG abnormalities in anterior visual pathway disease. Electroenceph clin Neurophysiol 1985; 61: S135.
Holder GE. Significance of abnormal pattern electroretinography in anterior visual pathway dysfunction. Br J Ophthalmol 1987a; 71: 166–71.
Mollon ID, Astell S, Reffin JP. A minimalist test of colour vision. In: Drum B, Moreland ID, Serra A, ed. Colour vision deficiencies vol X. Kluwer, Dordrecht 1991: 59–67.
Harding GFA, Crews SJ, Pitts SM. Psychophysical and visual evoked potential findings in hereditary optic atrophy. Trans Ophthalmol Soc UK 1979; 99: 96–102.
Holder GE. Abnormalities of the pattern ERG in optic nerve lesions: changes specific for proximal retinal dysfunction. in Barber, C. and Blum, T. eds. Evoked Potentials III, Butterworths, London, 1987b: 221–4.
Holder GE. The incidence of abnormal pattern electroretinography in optic nerve demyelination. Electroenceph clin Neurophysiol 1991a; 78: 18–26.
Berninger TA, Jaeger W, Krastel H. Electrophysiology and colour perimetry in dominant infantile optic atrophy. Br J Ophthalmol 1991; 75: 49–52.
Bach M, Gerling J, Geiger K. Optic atrophy reduces the pattern electroretinogram for both fine and coarse stimulus patterns. Clin Vis Sci 1992; 7: 327–34.
Berninger TA, Schuurmans RP. Spatial tuning of the pattern ERG across temporal frequency. Doc Ophthalmol 1985; 61: 17–25.
Holder GE. Pattern electroretinography in the evaluation of glaucoma and in optic nerve function. in Heckenlively, JR and Arden GB. eds. Principles and Practice of Clinical Electrophysiology of Vision, Mosby Year Book, St. Louis, 1991b: 549–56.
Ryan S, Arden GB. Electrophysiological discrimination between retinal and optic nerve disorders. Doc Ophthalmol 1988; 68: 247–55.
Sherman J. Simultaneous pattern reversal electroretinograms and visual evoked po-tentials in diseases of the macula and optic nerve. Ann NY Acad Sci 1982; 388: 214–226.
Harrison JM, O'Connor PS, Young RS, Kincaid M, Bentley R. The pattern ERG in man following surgical resection of the optic nerve. Invest Ophthalmol Vis Sci 1987; 28: 492–499.
Holder GE. The pattern electroretinogram in anterior visual pathway dysfunction and its relationship to the pattern visual evoked potential: A personal clinical review of 743 eyes. Eye 1997; 11: 924–34.
Maffei L, Fiorentii A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science 1981; 211: 953–5.
Tobimatsu S, Celesia G, Cone SB, Gujrati M. Electroretinograms to checkerboard pattern reversal in cats: physiological characteristics and effect of retrograde degeneration of ganglion cells. Electroenceph clin Neurophysiol 1989; 73: 341–52.
Rodieck RW. Which cells code for colour? In Valberg A and Lee BB. eds. From Pigment to Perception. Plenum. NY: 83–93.
Holder GE. Pattern electroretinography in patients with delayed pattern visual evoked potentials due to distal anterior visual pathway dysfunction. J Neurol Neurosurg Psychiat 1989; 52: 1364–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holder, G., Votruba, M., Carter, A. et al. Electrophysiological findings in Dominant Optic Atrophy (DOA) linking to the OPA1 locus on chromosome 3q 28-qter. Doc Ophthalmol 95, 217–228 (1998). https://doi.org/10.1023/A:1001844021014
Issue Date:
DOI: https://doi.org/10.1023/A:1001844021014