Abstract
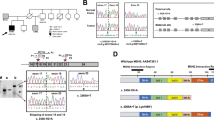
Hereditary non-polyposis colorectal cancer is a cancer predisposition syndrome known to be caused by heterozygous germline mutations in DNA mismatch repair genes (MMR) most commonly hMLH1, hMSH2, hMSH6. Heterozygous mutations in one of these genes confer an increased risk, mainly for colon and endometrial cancer. Recently, several publications identified that biallelic mutations in the MMR genes are associated with a more severe phenotype, including childhood malignancies and signs of neurofibromatosis type I (NF1). We report on a non-consanguineous Ashkenazi Jewish family with two affected siblings with features of NF1, colon cancer and astrocytoma at age 13 and 14. Their mother developed endometrial cancer at age 54. Their father had leukoplakia of the vocal cords with a family history of pancreatic cancer. Molecular and pathology studies were done on the tumor tissue and on genomic DNA of family members. Tumor testing demonstrated a high degree of microsatellite instability (MSI analysis), expression of MLH1 and absence of expression of both MSH2 and MSH6 proteins. A biallelic c.1906G > C (p.A636P) mutation in the hMSH2 gene was detected in the blood of one affected child. Parental genetic testing showed that each parent was heterozygote for the mutation. The c.1906G > C mutation is a founder mutation in the Ashkenazi Jewish population. To our knowledge this is the first report of homozygosity for this founder mutation.





Similar content being viewed by others
Abbreviations
- AC:
-
Amsterdam criteria
- CCS:
-
Childhood cancer syndrome
- CRC:
-
Colorectal cancer
- DHPLC:
-
Denaturing high performance liquid chromatography
- HNPCC:
-
Hereditary non-polyposis colorectal cancer
- IHC:
-
Immunohistochemistry
- MLPA:
-
Multiplex ligase dependent probe amplification
- MMR:
-
Mismatch repair
- MMR-D:
-
Mismatch repair deficiency
- MSI:
-
Microsatellite instability
- NF1:
-
Neurofibromatosis type 1
References
Lynch HT, Smyrk TC, Watson P et al (1993) Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 104:1535–1549
Vasen HF, Wijnen JT, Menko FH et al (1996) Cancer risk in families with hereditary nonpolyposis colorectal cancer diagnosed by mutation analysis. Gastroenterology 110:1020–1027. doi:10.1053/gast.1996.v110.pm8612988
Peltomaki P, Vasen HF (1997) Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology 113:1146–1158. doi:10.1053/gast.1997.v113.pm9322509
Boland CR (2000) Molecular genetics of hereditary nonpolyposis colorectal cancer. Ann N Y Acad Sci 910:50–59. discussion 59–61
De Vos M, Hayward BE, Picton S et al (2004) Novel PMS2 pseudogenes can conceal recessive mutations causing a distinctive childhood cancer syndrome. Am J Hum Genet 74:954–964. doi:10.1086/420796
Scott RH, Mansour S, Pritchard-Jones K et al (2007) Medulloblastoma, acute myelocytic leukemia and colonic carcinomas in a child with biallelic MSH6 mutations. Nat Clin Pract Oncol 4:130–134. doi:10.1038/ncponc0719
Wang Q, Lasset C, Desseigne F et al (1999) Neurofibromatosis and Early Onset of Cancers in hMLH1-deficient Children. Cancer Res 59:294–297
Menko FH, Kaspers GL, Meijer GA et al (2004) A homozygous MSH6 mutation in a child with café-au-lait spots, oligodendroglioma and rectal cancer. Fam Cancer 3:123–127. doi:10.1023/B:FAME.0000039893.19289.18
Krüger S, Kinzel M, Walldorf C et al (2008) Homozygous PMS2 germline mutations in two families with early-onset haematological malignancy, brain tumours, HNPCC-associated tumours, and signs of neurofibromatosis type 1. Eur J Hum Genet 16:62–72. doi:10.1038/sj.ejhg.5201923
de Wind N, Dekker M, Berns A et al (1995) Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell 82:321–330. doi:10.1016/0092-8674(95)90319-4
Reitmair AH, Schmits R, Ewel A et al (1995) MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet 11:64–70. doi:10.1038/ng0995-64
Reitmair AH, Redston M, Cai JC et al (1996) Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice. Cancer Res 56:3842–3849
Sohn KJ, Choi M, Song J et al (2003) Msh2 deficiency enhances somatic Apc and p53 mutations in Apc ± Msh2−/− mice. Carcinogenesis. 24(2):217–224
Feitsma H, Kuiper RV, Korving J et al (2008) Zebrafish with mutations in mismatch repair genes develop neurofibromas and other tumors. Cancer Res 68(13):5059–5066. doi:10.1158/0008-5472.CAN-08-0019
Nystrom-Lahti M, Kristo P, Nicolaides NC et al (1995) Founding mutations and Alu-mediated recombination in herediatry colon cancer. Nat Med 1:1203–1206. doi:10.1038/nm1195-1203
Yuan ZQ, Wong N, Foulkes WD et al (1999) A missense mutation in both hMSH2 and APC in an Ashkenazi Jewish HNPCC kindred: implications for clinical screening. J Med Genet 36:790–793
Foulkes WD, Thiffault I, Gruber SB et al (2002) The founder mutation MSH2*1906G → C is an important cause of hereditary nonpolyposis colorectal cancer in the Ashkenazi Jewish population. Am J Hum Genet 71:1395–1412. doi:10.1086/345075
Goldberg Y, Porat RM, Kedar I et al (2008) Mutation spectrum in HNPCC in the Israeli population. Fam Cancer (Apr):4
Vasen HF, Mecklin JP, Khan PM et al (1991) The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 34:424–425. doi:10.1007/BF02053699
Banjerjee SK, Maldisi WF, Weston AP et al (1995) Microwave-based DNA extraction from paraffin-embedded tissue for PCR amplification. Biotechniques 18(5):768–773
Fujita M, Enomoto T, Yoshino K et al (1995) Microsatellite instability and alterations in the hMSH2 gene in human ovarian cancer. Int J Cancer 64(6):361–366. doi:10.1002/ijc.2910640602
Shlush LI, Behar DM, Zelazny A et al (2002) Molecular epidemiological analysis of the changing nature of a meningococcal outbreak following a vaccination campaign. J Clin Microbiol 40(10):3565–3571. doi:10.1128/JCM.40.10.3565-3571.2002
Bercovich D, Beaudet AL (2003) Denaturing high-performance liquid chromatography for the detection of mutations and polymorphisms in UBE3A. Genet Test Fall 7(3):189–194
Bougeard G, Charbonnier F, Moerman A et al (2003) Early onset brain tumor and lymphoma in MSH2-deficient children. Am J Hum Genet 72:213–216. doi:10.1086/345297
Whiteside D, McLeod R, Graham G et al (2002) A homozygous germ-line mutation in the human MSH2 gene predisposes to haematological malignancy and multiple. Cancer Res 62:352–362
Puisieux A (1999) HNPCC syndrome, microsatellite instability and NF1 gene alteration (French). Bull Cancer Oct Rev 86(10):812–814
Wang Q, Montmain G, Ruano E et al (2003) Neurofibromatosis type 1 gene as a mutational target in a mismatch repair-deficient cell type. Hum Genet 112:117–123
Agostini M, Tibiletti MG, Lucci-Cordisco E et al (2005) Two PMS2 mutations in a Turcot syndrome family with small bowel cancers. Am J Gastroenterol 100(8):1886–1891. doi:10.1111/j.1572-0241.2005.50441.x
Hegde MR, Chong B, Blazo ME et al (2005) A homozygous mutation in MSH6 causes Turcot syndrome. Clin Cancer Res 11(13):4689–4693
Leach FS, Polyak K, Burrell M et al (1996) Expression of the human mismatch repair gene hMSH2 in normal and neoplastic tissues. Cancer Res 56(2):235–240
Thibodeau SN, French AJ, Roche PC et al (1996) Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 56(21):4836–4840
Ollila S, Sarantaus L, Kariola R et al (2006) Pathogenicity of MSH2 missense mutations is typically associated with impaired repair capability of the mutated protein. Gastroenterology 131(5):1408–1417. doi:10.1053/j.gastro.2006.08.044
Guillem JG, Moore HG, Palmer C et al (2004) A636P testing in Ashkenazi Jews. Fam Cancer 3(3–4):223–227. doi:10.1007/s10689-004-0899-z
Poley JW, Wagner A, Hoogmans MM et al (2007) Rotterdam Initiative on Gastrointestinal Hereditary Tumors. Biallelic germline mutations of mismatch-repair genes: a possible cause for multiple pediatric malignancies. Cancer 109(11):234
Acknowledgments
This work was supported, in part, by the Israeli Cancer Association, and by the Levinace Friedl foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toledano, H., Goldberg, Y., Kedar-Barnes, I. et al. Homozygosity of MSH2 c.1906G→C germline mutation is associated with childhood colon cancer, astrocytoma and signs of Neurofibromatosis type I. Familial Cancer 8, 187–194 (2009). https://doi.org/10.1007/s10689-008-9227-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-008-9227-3