Abstract
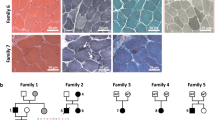
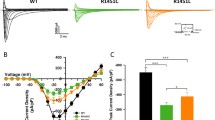
Muscle contraction upon nerve stimulation relies on excitation–contraction coupling (ECC) to promote the rapid and generalized release of calcium within myofibers. In skeletal muscle, ECC is performed by the direct coupling of a voltage-gated L-type Ca2+ channel (dihydropyridine receptor; DHPR) located on the T-tubule with a Ca2+ release channel (ryanodine receptor; RYR1) on the sarcoplasmic reticulum (SR) component of the triad. Here, we characterize a novel class of congenital myopathy at the morphological, molecular, and functional levels. We describe a cohort of 11 patients from 7 families presenting with perinatal hypotonia, severe axial and generalized weakness. Ophthalmoplegia is present in four patients. The analysis of muscle biopsies demonstrated a characteristic intermyofibrillar network due to SR dilatation, internal nuclei, and areas of myofibrillar disorganization in some samples. Exome sequencing revealed ten recessive or dominant mutations in CACNA1S (Cav1.1), the pore-forming subunit of DHPR in skeletal muscle. Both recessive and dominant mutations correlated with a consistent phenotype, a decrease in protein level, and with a major impairment of Ca2+ release induced by depolarization in cultured myotubes. While dominant CACNA1S mutations were previously linked to malignant hyperthermia susceptibility or hypokalemic periodic paralysis, our findings strengthen the importance of DHPR for perinatal muscle function in human. These data also highlight CACNA1S and ECC as therapeutic targets for the development of treatments that may be facilitated by the previous knowledge accumulated on DHPR.






Similar content being viewed by others
References
Al-Qusairi L, Laporte J (2011) T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet Muscle 1:26. doi:10.1186/2044-5040-1-26
Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ (2011) Prevalence of congenital myopathies in a representative pediatric united states population. Ann Neurol 70:662–665. doi:10.1002/ana.22510
Bevilacqua JA, Monnier N, Bitoun M, Eymard B, Ferreiro A, Monges S, Lubieniecki F, Taratuto AL, Laquerriere A, Claeys KG et al (2011) Recessive RYR1 mutations cause unusual congenital myopathy with prominent nuclear internalization and large areas of myofibrillar disorganization. Neuropathol Appl Neurobiol 37:271–284. doi:10.1111/j.1365-2990.2010.01149.x
Censier K, Urwyler A, Zorzato F, Treves S (1998) Intracellular calcium homeostasis in human primary muscle cells from malignant hyperthermia-susceptible and normal individuals. Effect of overexpression of recombinant wild-type and Arg163Cys mutated ryanodine receptors. J Clin Invest 101:1233–1242. doi:10.1172/JCI993
Chaudhari N (1992) A single nucleotide deletion in the skeletal muscle-specific calcium channel transcript of muscular dysgenesis (mdg) mice. J Biol Chem 267:25636–25639
Dowling JJ, Lawlor MW, Dirksen RT (2014) Triadopathies: an emerging class of skeletal muscle diseases. Neurotherapeutics 11:773–785. doi:10.1007/s13311-014-0300-3
Ducreux S, Zorzato F, Muller C, Sewry C, Muntoni F, Quinlivan R, Restagno G, Girard T, Treves S (2004) Effect of ryanodine receptor mutations on interleukin-6 release and intracellular calcium homeostasis in human myotubes from malignant hyperthermia-susceptible individuals and patients affected by central core disease. J Biol Chem 279:43838–43846. doi:10.1074/jbc.M403612200
Dulhunty AF, Karunasekara Y, Curtis SM, Harvey PJ, Board PG, Casarotto MG (2005) Role of some unconserved residues in the “C” region of the skeletal DHPR II-III loop. Front Biosci 10:1368–1381
Eberl DF, Ren D, Feng G, Lorenz LJ, Van Vactor D, Hall LM (1998) Genetic and developmental characterization of Dmca1D, a calcium channel alpha1 subunit gene in Drosophila melanogaster. Genetics 148:1159–1169
Engel AG, Franzini-Armstrong C (2004) Myology, basic and clinical. McGraw-Hill, New York
Fischer D, Herasse M, Bitoun M, Barragan-Campos HM, Chiras J, Laforet P, Fardeau M, Eymard B, Guicheney P, Romero NB (2006) Characterization of the muscle involvement in dynamin 2-related centronuclear myopathy. Brain 129:1463–1469. doi:10.1093/brain/awl071
Franzini-Armstrong C, Pincon-Raymond M, Rieger F (1991) Muscle fibers from dysgenic mouse in vivo lack a surface component of peripheral couplings. Dev Biol 146:364–376
Geoffroy V, Pizot C, Redin C, Piton A, Vasli N, Stoetzel C, Blavier A, Laporte J, Muller J (2015) VaRank: a simple and powerful tool for ranking genetic variants. PeerJ 3:e796. doi:10.7717/peerj.796
Hunter JM, Ahearn ME, Balak CD, Liang WS, Kurdoglu A, Corneveaux JJ, Russell M, Huentelman MJ, Craig DW, Carpten J et al (2015) Novel pathogenic variants and genes for myopathies identified by whole exome sequencing. Mol Genet Genomic Med 3:283–301. doi:10.1002/mgg3.142
Jurkat-Rott K, Lehmann-Horn F (2004) The impact of splice isoforms on voltage-gated calcium channel alpha1 subunits. J Physiol 554:609–619. doi:10.1113/jphysiol.2003.052712
Jurkat-Rott K, Lehmann-Horn F, Elbaz A, Heine R, Gregg RG, Hogan K, Powers PA, Lapie P, Vale-Santos JE, Weissenbach J et al (1994) A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet 3:1415–1419
Klein A, Jungbluth H, Clement E, Lillis S, Abbs S, Munot P, Pane M, Wraige E, Schara U, Straub V et al (2011) Muscle magnetic resonance imaging in congenital myopathies due to ryanodine receptor type 1 gene mutations. Arch Neurol 68:1171–1179. doi:10.1001/archneurol.2011.188
Knudson CM, Chaudhari N, Sharp AH, Powell JA, Beam KG, Campbell KP (1989) Specific absence of the alpha 1 subunit of the dihydropyridine receptor in mice with muscular dysgenesis. J Biol Chem 264:1345–1348
Kugler G, Weiss RG, Flucher BE, Grabner M (2004) Structural requirements of the dihydropyridine receptor alpha1S II–III loop for skeletal-type excitation–contraction coupling. J Biol Chem 279:4721–4728. doi:10.1074/jbc.M307538200
Kung AW, Lau KS, Fong GC, Chan V (2004) Association of novel single nucleotide polymorphisms in the calcium channel alpha 1 subunit gene (Ca(v)1.1) and thyrotoxic periodic paralysis. J Clin Endocrinol Metab 89:1340–1345. doi:10.1210/jc.2003-030924
Lu X, Xu L, Meissner G (1994) Activation of the skeletal muscle calcium release channel by a cytoplasmic loop of the dihydropyridine receptor. J Biol Chem 269:6511–6516
Malfatti E, Bohm J, Lacene E, Beuvin M, Romero NB, Laporte J (2015) A premature stop codon in MYO18B is associated with severe nemaline myopathy with cardiomyopathy. J Neuromuscul Dis 2:219–227. doi:10.3233/JND-150085
Malfatti E, Romero NB (2016) Nemaline myopathies: state of the art. Rev Neurol (Paris) 172:614–619. doi:10.1016/j.neurol.2016.08.004
Matthews E, Portaro S, Ke Q, Sud R, Haworth A, Davis MB, Griggs RC, Hanna MG (2011) Acetazolamide efficacy in hypokalemic periodic paralysis and the predictive role of genotype. Neurology 77:1960–1964. doi:10.1212/WNL.0b013e31823a0cb6
Monnier N, Procaccio V, Stieglitz P, Lunardi J (1997) Malignant-hyperthermia susceptibility is associated with a mutation of the alpha 1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet 60:1316–1325
Nance JR, Dowling JJ, Gibbs EM, Bonnemann CG (2012) Congenital myopathies: an update. Curr Neurol Neurosci Rep 12:165–174. doi:10.1007/s11910-012-0255-x
North KN (2011) Clinical approach to the diagnosis of congenital myopathies. Semin Pediatr Neurol 18:216–220. doi:10.1016/j.spen.2011.10.002
Pai AC (1965) Developmental genetics of a lethal mutation, muscular dysgenesis (Mdg), in the mouse. Ii. Developmental analysis. Dev Biol 11:93–109
Pfeffer G, Elliott HR, Griffin H, Barresi R, Miller J, Marsh J, Evila A, Vihola A, Hackman P, Straub V et al (2012) Titin mutation segregates with hereditary myopathy with early respiratory failure. Brain 135:1695–1713. doi:10.1093/brain/aws102
Pietri-Rouxel F, Gentil C, Vassilopoulos S, Baas D, Mouisel E, Ferry A, Vignaud A, Hourde C, Marty I, Schaeffer L et al (2010) DHPR alpha1S subunit controls skeletal muscle mass and morphogenesis. EMBO J 29:643–654. doi:10.1038/emboj.2009.366
Powell JA, Petherbridge L, Flucher BE (1996) Formation of triads without the dihydropyridine receptor alpha subunits in cell lines from dysgenic skeletal muscle. J Cell Biol 134:375–387
Ptacek LJ, Tawil R, Griggs RC, Engel AG, Layzer RB, Kwiecinski H, McManis PG, Santiago L, Moore M, Fouad G et al (1994) Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell 77:863–868
Rebbeck RT, Karunasekara Y, Board PG, Beard NA, Casarotto MG, Dulhunty AF (2014) Skeletal muscle excitation–contraction coupling: who are the dancing partners? Int J Biochem Cell Biol 48:28–38. doi:10.1016/j.biocel.2013.12.001
Rezgui SS, Vassilopoulos S, Brocard J, Platel JC, Bouron A, Arnoult C, Oddoux S, Garcia L, De Waard M, Marty I (2005) Triadin (Trisk 95) overexpression blocks excitation–contraction coupling in rat skeletal myotubes. J Biol Chem 280:39302–39308. doi:10.1074/jbc.M506566200
Romero NB, Clarke NF (2013) Congenital myopathies. Handb Clin Neurol 113:1321–1336. doi:10.1016/B978-0-444-59565-2.00004-6
Sekulic-Jablanovic M, Palmowski-Wolfe A, Zorzato F, Treves S (2015) Characterization of excitation–contraction coupling components in human extraocular muscles. Biochem J 466:29–36. doi:10.1042/BJ20140970
Susman RD, Quijano-Roy S, Yang N, Webster R, Clarke NF, Dowling J, Kennerson M, Nicholson G, Biancalana V, Ilkovski B et al (2010) Expanding the clinical, pathological and MRI phenotype of DNM2-related centronuclear myopathy. Neuromuscul Disord 20:229–237. doi:10.1016/j.nmd.2010.02.016
Tang ZZ, Yarotskyy V, Wei L, Sobczak K, Nakamori M, Eichinger K, Moxley RT, Dirksen RT, Thornton CA (2012) Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of Ca(V)1.1 calcium channel. Hum Mol Genet 21:1312–1324. doi:10.1093/hmg/ddr568
Treves S, Pouliquin R, Moccagatta L, Zorzato F (2002) Functional properties of EGFP-tagged skeletal muscle calcium-release channel (ryanodine receptor) expressed in COS-7 cells: sensitivity to caffeine and 4-chloro-m-cresol. Cell Calcium 31:1–12
Wu J, Yan Z, Li Z, Yan C, Lu S, Dong M, Yan N (2015) Structure of the voltage-gated calcium channel Cav1.1 complex. Science 350:aad2395. doi:10.1126/science.aad2395
Zorzato F, Scutari E, Tegazzin V, Clementi E, Treves S (1993) Chlorocresol: an activator of ryanodine receptor-mediated Ca2+ release. Mol Pharmacol 44:1192–1201
Acknowledgements
We thank Isabelle Marty for triadin antibody, John Rendu for RYR1 molecular testing, Anne-Sophie Nicot and Clara Franzini-Armstrong for discussions, Robert Y. Carlier for analysis of MRI images, and Nicola Foulds for clinical discussions. This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), University of Strasbourg, the France Génomique National infrastructure, funded as part of the Investissements d’Avenir program managed by the Agence Nationale pour la Recherche (ANR-10-INBS-09), and by Fondation Maladies Rares within the frame of the “Myocapture” sequencing project, ANR-10-LABX-0030-INRT under the frame program Investissements d’Avenir ANR-10-IDEX-0002-02, Association Française contre les Myopathies (AFM-17088), Muscular Dystrophy Association (MDA-186985), Myotubular Trust and Sparks the Children’s medical research charity grant N° 12KCL 01-MT, and the Swiss National Science Foundation grant N° 31003A-146198. T.M.P. was supported by the Diana and Steve Marienhoff Fashion Industries Guild Endowed Fellowship in Pediatric Neuromuscular Diseases. F.M. is supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London. The Biobank of the MRC Neuromuscular Centre and the support of the Muscular Dystrophy UK to the Dubowitz Neuromuscular Centre are also gratefully acknowledged.
Web resources
ExAC Browser/Exome Aggregation Consortium (URL: http://exac.broadinstitute.org/). Exome Variant Server (URL: http://evs.gs.washington.edu/EVS/) [March, 2012]). 1000 genomes (URL: http://www.1000genomes.org/). Database of Single Nucleotide Polymorphisms (dbSNP Build ID: 134) (URL: http://www.ncbi.nlm.nih.gov/SNP/). Online Mendelian Inheritance in Man (OMIM) (URL: http://www.omim.org/).
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Schartner and N. B. Romero contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schartner, V., Romero, N.B., Donkervoort, S. et al. Dihydropyridine receptor (DHPR, CACNA1S) congenital myopathy. Acta Neuropathol 133, 517–533 (2017). https://doi.org/10.1007/s00401-016-1656-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-016-1656-8