Article Text
Abstract
Background Leucocyte telomere length (LTL) is a complex trait associated with ageing and longevity. LTL dynamics are defined by LTL and its age-dependent attrition. Strong, but indirect evidence suggests that LTL at birth and its attrition during childhood largely explains interindividual LTL variation among adults. A number of studies have estimated the heritability of LTL, but none has assessed the heritability of age-dependent LTL attrition.
Methods We examined the heritability of LTL dynamics based on a longitudinal evaluation (an average follow-up of 12 years) in 355 monozygotic and 297 dizygotic same-sex twins (aged 19–64 years at baseline).
Results Heritability of LTL at baseline was estimated at 64% (95% CI 39% to 83%) with 22% (95% CI 6% to 49%) of shared environmental effects. Heritability of age-dependent LTL attrition rate was estimated at 28% (95% CI 16% to 44%). Individually unique environmental factors, estimated at 72% (95% CI 56% to 84%) affected LTL attrition rate with no indication of shared environmental effects.
Conclusions This is the first study that estimated heritability of LTL and also its age-dependent attrition. As LTL attrition is much slower in adults than in children and given that having a long or a short LTL is largely determined before adulthood, our findings suggest that heritability and early life environment are the main determinants of LTL throughout the human life course. Thus, insights into factors that influence LTL at birth and its dynamics during childhood are crucial for understanding the role of telomere genetics in human ageing and longevity.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Leucocyte telomere length (LTL) is a complex human trait; it is heritable,1–5 longer in women than in men6–8 and longer in offspring of older fathers.9–12 A body of research also shows that LTL might be modified by environmental factors, including smoking,5 ,13 ,14 body mass index (BMI),13–15 energy intake16 and sedentary lifestyle.17 ,18 In line with LTL heritability, recent genome-wide association studies have begun to decipher genes that explain some of the interindividual variation in LTL in the general population.19–22 LTL dynamics are defined by two parameters: LTL at birth and its age-dependent attrition afterward.23 ,24 The age-dependent LTL attrition ostensibly reflects haematopoietic stem cell replication,24–27 because telomerase activity in these cells is insufficient to prevent telomere attrition due to replication.28–30
While information is available about the effect of heritability on LTL, little is known about whether heritability also impacts the rate of LTL attrition. This information is highly relevant, given that LTL has been linked with longevity31–34 and ageing-related cardiovascular disease, principally in the form of atherosclerosis.35 In the present longitudinal study, using the same-sex twin model, we examined how heritability and the environment affect the absolute LTL and also the rate of age-dependent LTL attrition in participants of the GEMINAKAR study.36 ,37
Methods
Design and study population
Twin pairs, aged 19–64 years at baseline examination, were recruited in two investigative sites set up in Odense and in Copenhagen, to participate in a longitudinal study of metabolic disorders and cardiovascular risk factors. Recruitment was through the National Danish Twin Registry.36 Baseline examination was performed between 1997 and 2000, while follow-up examination was conducted between 2010 and 2012.
The cohort, named GEMINAKAR, consisted of twin pairs without history of diabetes or cardiovascular disease at baseline examination. They were subjected to baseline physical examination, venous puncture for fasting blood samples and the collection of a comprehensive anthropometric and demographic data. At the follow-up examination, the twins were visited at home or at work by a mobile examination unit, which included a research nurse and a laboratory technician. The evaluation carried out in the mobile examination unit was comparable with that of the baseline examination.37 Zygosity was determined at baseline by the Institute of Forensic Genetics in Copenhagen, Denmark, using the same set of DNA-based microsatellite markers as for paternity cases with the PE Applied Biosystems AmpFISTR Profiler Plus Kit (PE Applied Biosystems, Foster City, California, USA). As per previous work,31 we have focused, in this study, on a same-sex twin model. All participants provided written informed consent.
Leucocyte telomere length measurements
LTL measurements were performed as previously described.38 Briefly, DNA was extracted from thawed buffy coats using the salting-out method as described by Miller39 and integrity assessed by resolving samples on 1% (wt/vol) agarose gel. Samples were digested with restriction enzymes Hinf I (10 U) and Rsa I (10 U; Roche). The analysis of the terminal restriction fragments was performed in duplicate (on different gels and occasions). Samples of the cotwins in each twin pair were randomised. However, baseline and follow-up DNA samples from each twin were resolved in adjacent lanes on 0.5% agarose gels. After 16 h, the DNA was depurinated for 15 min in 0.25 N HCl, denatured for 30 min in 0.5 M NaOH/1.5 mol/L NaCl and neutralised for 30 min in 0.5 mol/L Tris, pH 8/1.5 M NaCl. The DNA was transferred for 1 h to a positively charged nylon membrane (Roche) using a vacuum blotter (Boeckel Scientific, Feasterville, Pennsylvania, USA). Membranes were hybridised at 65°C with the digoxigenin (DIG)-labelled telomeric probe overnight. The DIG-labelled probe was detected by the DIG luminescent (Roche) and exposed on X-ray film. The interassay coefficient of variation of the TL measurements was 1.3%. Insufficient DNA or degraded DNA precluded measurements of LTL in a small subset of follow-up blood samples (table 1).
Characteristics of the twins
Modelling of telomere attrition in twin pairs
The outcome of LTL with associated factors, namely, age, sex, BMI and smoking status, was analysed in the following settings: (A) cross-sectional (baseline LTL and follow-up LTL, with adjustment for covariates) and (B) longitudinal (difference in LTL between follow-up and baseline examinations, and LTL at follow-up adjusted for LTL at baseline and covariates). Within-pair dependence in LTL in the above settings was assessed by the (intraclass) correlation coefficient. This correlation was obtained from random effects regression of LTL on the covariates and a random pair-specific intercept, allowing for decomposing the variation in LTL into between-pair and within-pair variation. For the study of LTL attrition between baseline and follow-up examinations, an individual-specific intercept was further added as described previously.40
Quantitative genetic models were analysed to estimate the magnitude of genetic and environmental influences41 ,42 that explained variance in the absolute LTL or LTL attrition. Heritability was defined as the proportion of variance in LTL or LTL attrition due to genetic factors. The general approach analysed covariance of LTL or LTL attrition between cotwins of monozygotic (MZ) and dizygotic (DZ) pairs to decompose the LTL or LTL attrition into a sum of components: A (additive genetic effects), D (dominant genetic effects, which model deviations of the heterozygote genotype from the mean of the homozygote genotype), C (common, ie, shared, environmental effects) and E (individually unique environmental effects). The genetic parameters of the model were specified based on the biological relationship between the cotwins. Within-pair covariance of LTL was expressed as κ var (A)+γ var (D)+var (C), where κ=γ=1 for MZ pairs and κ=1/2 and γ=1/4 for DZ pairs.41 ,42
A, D and C cannot be estimated simultaneously.41 Therefore, a series of models were tested which allowed for sequential testing of the significance of specific parameters. Measurement error was estimated in E, as this is the component of variance that does not contribute to within-twin pair resemblance. Dominance effects are typically biologically implausible in the absence of additive effects. The primary models were thus the ACE and ADE models, as well as their sub-models AE, CE and E. We assessed the fit of the models relative to the saturated model by the χ2 statistics and used the Akaike information criterion for comparison of models.42 We report the within-pair correlation for MZ and DZ pairs, the heritability, dominant genetic, shared environment and unique environmental effects for each of the above models. All estimates are adjusted for effects of age at baseline and sex. Covariates that were not significant at 10% level were left out of the analysis. We modelled the level (intercept) and change (slope) separately. The bivariate growth curve model for the joint intercept and slope, for instance, incorporates genetic pleiotropy—the genetic correlation of intercept with the slope. However, in case of having only two measurements for each individual, the slope marginal of the growth curve model is equivalent to modelling the difference, including the error term as we described above.
Results
General characteristics of the twins at baseline and follow-up examinations are displayed in table 1.
At baseline, LTL showed attrition across individuals of different ages at −0.022 kb/year (p<0.001). On average, women had a longer LTL than men (0.16 kb, p<0.01) (table 2). Based on the longitudinal data, the yearly rate of LTL attrition was estimated at −0.020 kb/year (p<0.001). LTL did not differ between MZ and DZ twins (p=0.50). Similarly, based on the longitudinal data, LTL attrition did not differ between MZ and DZ twins (table 2).
Factors influencing leucocyte telomere length
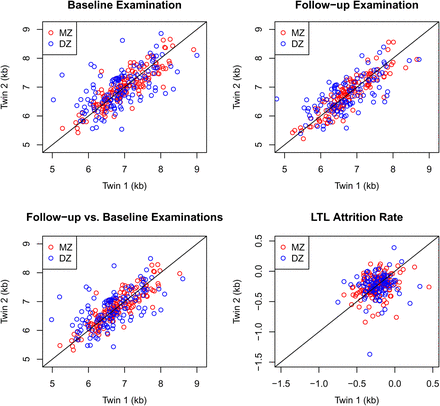
We analysed the covariance of LTL or LTL attrition between cotwins of MZ and DZ pairs by decomposing LTL or LTL attrition into a sum of components: A, D, C and E. As shown in table 3, when adjusting for sex and age at baseline examination, the models AE, ACE and ADE yielded estimates of LTL heritability between 46% and 87% with no indication of dominant genetic effects. The model with solely environmental effects (CE model) displayed very poor fit to data. By contrast, the ACE model gave the most parsimonious fit to data at baseline and at follow-up, with estimated heritability of LTL at baseline of 64% (95% CI 39% to 83%) and significant 22% (95% CI 6% to 49%) of shared environmental effects. These results were robust to stratification into age groups and birth cohorts, and were similar and not significantly different than those obtained for LTL at follow-up examination in which the ACE model also gave the most parsimonious fit. Figure 1 displays the data points in four twin–twin plots corresponding to table 3. This shows how correlated the pairs tend to be depending on zygosity for baseline, follow-up, follow vs baseline, which is the predicted LTL at follow-up based on baseline LTL, as predicted by the best fitting AE model and the LTL attrition, which is the difference between follow-up and baseline LTLs. Comparisons of correlations between MZ and DZ twins of LTL stratified by quartiles showed no evidence for different degrees of heritability of shorter versus longer LTLs.
Biometrics of leucocyte telomere length dynamics
Twin-twin plots of the four LTL measures modelled in table 3. The follow-up versus baseline examinations plot presents the predicted LTL values from the best fitting AE model. The diagonal line represents perfect correlation. LTL, leucocyte telomere length; AE, sub-model of ACE with zero shared environmental component; DZ, dizygotic twins; MZ, monozygotic twins.
For the LTL attrition rate, the AE, ACE and ADE models yielded very similar estimates of heritability between 24% and 32% with estimated non-significant 7% of shared environmental effects and no indication of dominant genetic effects. The model with solely environmental effects (CE model) fitted very poorly to data, while the AE model gave the most parsimonious fit with estimated heritability of 28% (95% CI 16% to 44%) and unique environmental effects of 72% (95% CI 56% to 84%). These results were also observed for the outcome of LTL adjusted for baseline in which the AE model gave the most parsimonious fit to the data yielding very similar estimates.
Discussion
In this study, we show that the LTL is substantially heritable, as has been shown before, where the reported heritability of LTL ranged between 36% and 82%.1–5 ,8 Notably, we also show that the rate of LTL attrition during adult life is heritable, although to a lesser extent than LTL, with an indication of low or no shared environmental effect.
LTL dynamics during the first 20 years of life exert an outsize effect on LTL for the remaining life course because of the wide range of LTL across newborns43 ,44 and the fast rate of LTL attrition during growth and development.23–26 By contrast, the overall effect of LTL attrition during adult life on LTL is relatively small. Thus, by the age of 20 years, most adults display fixed ranking and tracking of LTL, such that those individuals with a relatively short (or long) LTL as young adults are likely to display a relatively short (or long) LTL as they get older.45 In this light, it might be relevant to consider the nature and impact of environmental factors that largely influence LTL at birth and during growth and development versus those that affect its age-dependent attrition during adulthood.
The predominant environmental factors that influence LTL are those shared by cotwins in each twin pair. By contrast, essentially, only individually unique environmental factors influence the LTL attrition rate during the follow-up period and, presumably, throughout adult life course. What might be the biological meaning for these findings?
Shared environmental factors that impact adult LTL primarily reflect the first two decades of life, unless the cotwins are raised apart. The principal environmental factors that are shared by the cotwins might start in utero and extend to the extrauterine life—primarily the period of growth and development. Given that the extrauterine period of growth and development is marked by a rapid rate of LTL attrition, the influence of the shared environment of the cotwins during this time might exert a further lasting effect on LTL for the remaining life course. Thus, in absolute terms, the effect on LTL by shared environmental factors is much larger than that exerted by individually unique environmental factors, which largely influence the rate of LTL attrition during adulthood. In this context, in the GEMINAKAR study, females of opposite-sex twins were found to have LTL that was equivalent to their male cotwins.46 Although the aetiology of the ablated sex difference in LTL in opposite-sex twins is unknown, it might stem from the influence of the male fetus on the female fetus telomere dynamics in utero, or the shared environment of the cotwins during early extrauterine life.46 To avoid the confounding effect of opposite-sex twins on LTL, the present study was limited to same-sex twins.
The approach taken in the analysis of the genetic influence on LTL level and LTL attrition was to consider each separately. The full bivariate growth curve model for level and change is, however, fragile when conditioning on natural assumptions for twin pairs, but in case of having only two measurements for each individual, the slope (change) marginal of this model is equivalent to modelling the difference, as we do. We note that when regressing the follow-up outcome on the baseline (table 3) which is preferred for this design, we also gain a degree of freedom in comparison to studying the difference.
A host of individually unique environmental factors, such as energy intake,16 lifestyle,17 ,18 socioeconomic status47 and mental stress48 might differently impact the rate of LTL attrition in cotwins during adult life. However, as shown in the present study, the overall effect of these factors is relatively small compared with the joint effect on LTL of heritability and shared environmental factors, which is estimated at ∼87%.
In conclusion, the same-sex twin model points to heredity and shared environmental factors which are likely to exert their effect in utero and during early extrauterine life primarily through epigenetic modalities.49 Additionally, individually unique environmental factors exert a major effect on the rate of LTL attrition during adulthood. However, when set against the wide interindividual variation in LTL at birth and the rapid pace of LTL attrition during childhood, individually unique environmental factors appear to have only a small effect on LTL in adults. Understanding the role of genetics and the environment in fashioning LTL at birth and its attrition during childhood might hold the key for gaining insight into the role of telomere biology in human ageing and longevity.
References
Footnotes
-
Contributors JBH, TS and SM performed the heritability analysis. CD and KOK oversaw the data acquisition. MK oversaw telomere measurements. KC and AA obtained funding and designed the overall study. All authors contributed to the writing of the manuscript.
-
Funding This work was supported by a National Institutes of Health grant (AG030678); the Danish Council for Independent Research—Medical Sciences; the INTERREG 4 A—programme Southern Denmark-Schleswig-K.E.R.N. supported by the European Regional Development Fund; and the A.P. Møller Foundation for the Advancement of Medical Science.
-
Competing interests None.
-
Ethics approval Danish Ethics Committee and Danish Data Protection Board.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Data sharing statement Telomere data from this NIH-funded study are available for all interested researchers.