Article Text
Statistics from Altmetric.com
Periodontitis, a chronic inflammation of the tissues surrounding the teeth, is a common disease affecting all populations. The main aetiology remains a bacterial infection that leads to gingival inflammation, loss of alveolar bone, and tooth loss.1 Although the presence of pathogenic micro-organisms is required to trigger this process, the amplification and progression of the disease is believed to rely heavily on the production of host mediators in response to bacteria and/or their metabolic products.2 The CD14 molecule, described as the major endotoxin receptor, is one of the receptors which act on the recognition of lipopolysaccharides (LPS, endotoxin) and gram positive or mycobacterial cell wall components and thus can initiate the innate immune response to bacterial invasion.3–5 It is constitutively expressed primarily on the surface of monocytes, macrophages, neutrophils, and gingival fibroblasts (mCD14).6 In addition, a soluble form of CD14 (sCD14) is abundant in serum and is apparently derived both from the secretion of CD14 and from enzymatically cleaved glycosyl-phosphatidylinositol anchored mCD14.7 Besides the role of CD14 in the host defence, several other biological functions have been found. CD14 is involved in the phagocytosis of gram negative bacteria,8 LPS mediated bone resorption,9 and monocyte-endothelial cell interactions. Furthermore, changes in CD14 expression and serum sCD14 levels seem to be associated with a number of pathological states including periodontal diseases.10
CD14 production is genetically regulated. The gene for the CD14 receptor is on chromosome 5 (region q23-21), consists of ≈3900 bp organised in two exons, and encodes a protein of 375 amino acids.11 In the promoter region of the CD14 gene, a C to T transition was identified at position –159 upstream from the major transcription site, which is near to an SP1 binding site that has a major influence on the monocyte specific expression of CD14, and near a CCAAT/enhancer binding protein site that may play an important role in the promoter activation of the CD14 gene during monocyte development.12,13 Recent observations suggest that the TT homozygotes for this gene have a significantly increased density of CD14 in blood monocytes and increased serum levels of CD14 (sCD14) when compared with those involving the CT or the CC genotype.14,15 More recently, four additional SNPs within 2 kb of the CD14 transcription start sites, at positions –1619, –1359, –1145, and –809, have been identified.16 Carriers of the –1359GG/–1145AG/–159CT and –1359GT/–1145AG/–159CT haplotypes had a higher sCD14 than any other groups and the group with haplotypes –1359TT/–1145AA/–159CC had the lowest concentrations of sCD14.17 These findings suggest that the individual CD14 genotypes may be relevant in conditions where CD14 levels are increased, as in periodontitis.10
Considering the previously reported data, selected functional CD14 promoter polymorphisms were analysed in periodontitis patients compared to healthy controls in order to explore if CD14 genetic variances may be involved in the susceptibility and/or severity of chronic periodontitis.
Key points
-
Chronic periodontitis, which is the major cause of tooth loss in adults, is characterised by a chronic inflammation of periodontal tissues mainly caused by infection with subgingival, gram negative, anaerobic bacteria. Cellular responses in periodontitis are mediated, in part, by bacterial lipopolysaccharide (LPS), which activates monocytes to express cytokines, growth factors, and procoagulatory factors via LPS receptor CD14.
-
The aim of this study was to determine whether the functional polymorphisms in the promoter of the CD14 gene are associated with the risk of chronic periodontitis and the severity of the disease. Two polymorphisms located in the promoter region (C(−159)T and G(−1359)T) were analysed by PCR and RFLP methods in a group of 135 patients with chronic periodontitis, and in 207 age and sex unrelated, randomly selected control subjects.
-
The CD14 allele and genotype distributions were similar in patients and controls. However, the frequency of the G allele of the G(−1359)T polymorphism was higher in patients with severe disease than moderate disease (p<0.01, pcorr<0.05). The homozygous genotype (GG) of this polymorphism was found to be significantly increased in the severe patient group (p<0.01, pcorr< 0.05).
-
Allele and/or genotype frequencies of the C(−159)T polymorphism did not differ significantly between severe and moderate periodontitis patients. However, we detected a tendency for an increased frequency of the –159TT homozygotes in severe patients (19.2%) compared with the moderate subgroup (8.3%). In one patient, we identified a new mutation (28 bp deletion) in the promoter of the CD14 gene (del from –1310 to –1337), which occurred in a heterozygous combination.
-
This study shows that a specific genetic marker, which was previously associated with an increased sCD14 level, may act as a disease modifier of chronic periodontitis in the population investigated.
METHODS
Study subjects
All subjects were Europid of exclusively Czech ethnicity, free of all systemic diseases (especially cardiovascular disorders, such as coronary heart disease (CHD) and hypertension, diabetes mellitus, or allergy) and were not taking any medication. Phenotype status was assigned without knowledge of the genotypes by two independent investigators. One hundred and thirty-five Czech patients with chronic periodontitis (69 male and 66 female, age range 35-50 years, mean age 42.5 (SD 7.5) years) referred to the Periodontal Clinic of the Masaryk University St Anne Hospital were included in the study. Subjects were screened using a WHO probe and the CPITN (Community Periodontal Index of Treatment Needs) was assessed.18 All patients fulfilled the diagnostic criteria defined by the International Workshop for a Classification of Periodontal Diseases and Conditions for chronic periodontitis.19 The control population consisted of 207 unrelated randomly selected white subjects (112 male and 95 female, mean age 45 (SD 5.2) years) residing in the same geographical area as the patients, who did not have a clinical history of periodontal disease.
Clinical assessments
Clinical assessments of the patients were performed by the same investigator at the patient’s first visit. The diagnosis of chronic periodontitis was based on medical and dental history. The assessed clinical parameters were: probing pocket depth (PPD), clinical attachment loss (CAL), tooth mobility, and radiographs. PPD (from the free gingival margin to the bottom of the pocket) and CAL (from the cement enamel junction to the bottom of the pocket) of all teeth were assessed by using a probe at four sites/tooth: mesiobuccal (mb), distobuccal (db), mesiolingual (ml), and distolingual (dl). The loss of alveolar bone was determined radiographically. Full mouth radiographs of diagnostic quality were evaluated by a single calibrated reader for interproximal bone loss. We used the index of Mühlemann and Mazor20 to evaluate decreases in alveolar bone level. In all patients, extensive alveolar bone loss (≥25%) was determined.
Patients were classified according to the severity of their periodontal disease (on the basis of the amount of clinical attachment loss (CAL) and bone loss)21 into one of three disease categories. The “mild” (n=0) classification required no CAL >2 mm and no sites with bone loss >25% (n=0). The “moderate” (n=36) classification required 3 to 5 mm CAL and <5 interproximal sites with ≥50% bone loss. The “severe” (n=99) classification required ≥6 mm CAL and ≥5 interproximal sites with ≥50% bone loss.
Smoking history
In order to adjust for the effect of smoking history on periodontal disease, the subjects (patients and controls) were classified into the following groups: subjects who had never smoked (referred to as non-smokers), subjects who were former smokers for ≥5 pack years (referred to as former smokers), or current smokers (smokers). These comprised: light smokers (subjects smoking ≤10 cigarettes per day for <5 pack years), moderate smokers (subjects smoking >10 cigarettes per day for ≥5 pack years and <10 pack years), and heavy smokers (subjects smoking >10 cigarettes per day for ≥10 pack years). Pack years were calculated by multiplying the number of years smoked by the average number of cigarette packs smoked per day.22
Informed consent was obtained from all participants. The study was performed with the approval of the ethics committee of the Medical Faculty, Masaryk University, Brno.
Genotype identification
Genomic DNA was isolated from peripheral blood leucocytes by a standard method using the proteinase K digestion of cells according to Sambrook et al.23
Detection of the biallelic polymorphism C(−159)T in the promoter of the CD14 gene
The C(−159)T polymorphism was detected using a modification of a method described previously.15 A 497 bp PCR product was generated using primers 5‘-GTGCCAACAGATGAGGTTCAC-3‘ and 5‘-GCCTCTGACAGTTTATGTAATC-3‘. Commercially available AvaII endonuclease (New England Biolabs) is specific for the sequence GGTCC, which is present in the PCR product only among carriers of the CD14/−159T allele. The digested fragments were separated by electrophoresis on a 2% agarose gel and visualised with ethidium bromide staining. The homozygous C allele of the CD14 gene appears as one 497 bp band and the homozygous T allele as 144 and 353 bp fragments. Heterozygotes exhibited all bands of 144, 353, and 497 bp.
Detection of the biallelic polymorphism G(−1359)T in the promoter of the CD14 gene
The G(−1359)T polymorphism was detected by newly developed PCR methods and a subsequent restriction analysis with FokI endonuclease (New England Biolabs). Specific primer sequences (5‘-GTTGCAGTGAGCCAAGATCA-3‘ and 5‘-CCCTAGACCTCTGGGGAAAG-3‘) were derived from the original sequence (GenBank, Accession No X74984). The PCR was performed in a final volume of 15 μl, containing 50 mmol KCl, 10 mmol/l Tris-HCl buffer (pH 8.4), 1.7 mmol/l MgCl2, 6 pmol/l of each primer, 200 μmol/l dNTP, and 0.1 μg of genomic DNA in the presence of 0.1 U Taq polymerase (MBI Fermentas) to provide products of 120 bp. After the initial denaturation step (95°C for two minutes), each cycle (of an additional 30) consisted of a 94°C denaturation for 20 seconds, a 61°C annealing for 10 seconds, a 72°C extension for 15 seconds, with the final extension lasting for five minutes at 72°C. Twelve μl of the PCR product were digested with FokI for two hours at 37°C. The digestion showed fragments of 96 and 24 bp for the T allele, and 120 bp for the G allele.
Sequence analysis
PCR was performed with G(−1359)T primers as described above. PCR products were separated on a 3% agarose gel and atypical fragments were used as sequencing templates. Sequence analysis was performed by the cycle sequencing method, using the Thermo Sequence radiolabelled terminator cycle sequencing kit (Amersham, UK) and Redivue 33P-labelled terminator pack (Amersham, UK). The sequencing reaction was performed according to the manufacturer’s protocol. The electrophoresis was run on a BioRad sequencing apparatus in 6% polyacrylamide, 8 mol/l urea at constant 22 mA for two hours. We confirmed sequence identity of the novel mutation by performing sequencing reactions in both directions. In addition, the same deletion was observed in two different PCR products from the same DNA sample.
Statistical analysis
Allele frequencies were calculated from the observed numbers of genotypes. The significance of differences in allelic frequencies between each group was determined by Fisher’s exact test. χ2 analysis was used to test for a deviation of genotype distribution from Hardy-Weinberg equilibrium and for a comparison of differences in genotype combinations among groups. Contingency table analysis, odds ratio (OR), 95% confidence intervals, and significance values were estimated with the use of the program package Statistica v 3.0 (Statsoft Inc, Tulsa, USA). Corrections for multiple comparisons (Holm’s method) were performed and only the values of pcorr (=p corrected) less than 0.05 were considered to be significant.24
RESULTS
Distribution of CD14 genotypes and alleles in periodontitis patients and healthy controls
The distributions of the CD14 genotype and allele frequencies in patients with chronic periodontitis and age matched healthy controls was assessed. The frequencies of C(−159)T CD14 CC, −159CT, and –159TT were 29.5 %, 51.2 %, and 19.3 % in the controls and 25.9%, 57.8%, and 16.3% in the total patient group. The frequencies of G(−1359)T CD14 GG, −1359GT, and –1359, TT were 58.0 %, 38.2%, and 3.8% in the controls, and 56.7%, 40.3%, and 3.0% in the periodontitis group. No skewing was found in the distribution of the CD14 genotypes between patients and controls (table 1). Similarly, the CD14 allele frequency did not differ between the two groups (Fisher’s exact test, p= 0.13 and p=0.51, respectively).
Distribution of the CD14 genotypes and alleles in patients with chronic periodontitis and controls
Distribution of CD14 genotypes and alleles in periodontitis patients with moderate and severe disease
We next studied whether there was a relationship between the CD14 genotype and allele frequencies and the severity of periodontitis. Distributions were compared between 36 Czech chronic periodontitis patients with moderate disease and 99 patients with severe disease. A significant skewing was observed in the distribution of the CD14 (−1359G/T) genotypes between these subgroups. Homozygosity for the CD14 –1359 G allele was found in 63.3% of the 98 patients with severe disease compared to 38.9% of the 36 patients with moderate disease (table 2). Similarly, the CD14 allele frequency differed between the two groups. In the patients with severe disease, there was a relative overrepresentation of the CD14 –1359G allele (Fisher’s exact test, p=0.009, pcorr <0.05) (odds ratio GG v GT+TT genotypes: 2.71, 95% confidence interval (CI) 1.23 to 5.94).
Distribution of CD14 genotypes and alleles in Czech periodontitis patients with moderate and severe disease
Concerning the –159C/T genotypes, there were no significant differences between the severe and moderate subgroups of patients with chronic periodontitis. However, we detected a tendency for an increased frequency of the –159TT homozygotes in the severe patients (19.2%) compared with the moderate subgroup (8.3%) (p=0.10) (odds ratio TT v CC+CT genotypes 2.61, 95% confidence interval (CI) 0.72 to 9.43).
Detection of a novel mutation in the promoter of the CD14 gene
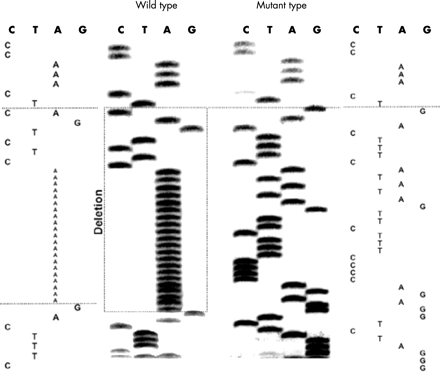
Gel separation of the PCR product of one patient showed an abnormal banding pattern. Applying a direct sequencing technique showed the existence of a novel variant (EMBL, accession No AJ491310) in the promoter of the CD14 gene with a deletion of 28 bp (aaaaaaaaaaaaaaaaaaaaactctgac) from position –1310 to –1337 bp (fig 1), which occurred in a heterozygous combination. It is probably a rare mutation with a frequency of less than 1% in the present population.
New mutation (28 bp deletion) in the promoter of the CD14 gene (del from –1310 to –1337).
Distribution of CD14 genotypes and alleles in periodontitis patients and healthy controls according to smoking status
Smoking was suggested to modify associations between a number of genes in chronic periodontitis, so the influence of an interaction between CD14 polymorphisms and the smoking status on periodontitis risk was investigated.25,26 No significant differences between smokers and non-smokers and/or between different categories of smoking severity were found for either polymorphism (data not shown).
DISCUSSION
The pathogenesis of chronic periodontitis remains poorly understood and it is now seen as a complex interplay between bacterial infection and host response, often modified by behavioural factors. In view of the possible role of the CD14 gene in the pathogenesis of chronic periodontitis, we analysed functional promoter polymorphisms of the CD14 gene in association with disease susceptibility and severity.
In spite of systematic analysis, the CD14 distribution did not differ between Czech periodontitis patients and age matched, unrelated, randomly selected controls, indicating that CD14 genotypes do not, per se, constitute a risk factor for the development of chronic periodontitis. However, a significant difference between the CD14 allele frequencies and the severity of chronic periodontitis was detected. Carriers of the CD14/−1359 G allele were more frequent among the patients with severe periodontitis than in the moderate periodontitis group. The odds ratio for severe disease associated with genotype GG v GT+TT was estimated as 2.71. There was a trend towards over-representation of the “more active” –159TT genotype (and T allele), which can decrease the affinity of Sp protein binding (it results in decreased affinity of DNA/protein interactions at a GC box27) and enhances transcriptional activity in patients with severe chronic periodontitis compared with patients with moderate disease. There are several plausible explanations for a possible role of CD14 in periodontal disease. Firstly, −1359G (eventually –159T) may be the functional variant in the CD14 association with periodontitis severity. Since the variant is within the promoter region of the CD14 gene, it may act to regulate its transcription. The association is supported by the previous finding10 that concentrations of sCD14 in serum were higher in patients with chronic periodontitis than in healthy subjects. CD14 on monocytes and polymorphonuclear cells functions as a receptor for LPS, thereby inducing mediator and cytokine release,28 including interleukin-1, TNF-α, and interleukin-6, which have been found in increased amounts in periodontitis patients. Blockade of CD14 prevents the release of LPS induced cytokines.29 Thus, CD14 may be involved in a proinflammatory pathway through the release of cytokines. Secondly, the difficulty with any candidate gene analysis in a disorder associated with a complex genetic trait is the potential effect of linkage disequilibrium. It is therefore possible that this candidate gene is in linkage disequilibrium with the actual causative gene. Finally, the CD14 contribution to periodontitis severity may comprise an interaction of several genes with an additional influence of environmental factors.
There is no doubt that specific bacteria initiate periodontitis.30 Since the CD14 genetic factor is involved in immuno-inflammatory processes, the clinical effects of this genetic factor depend on the presence of bacteria to initiate the inflammation. Therefore, patients with this genetic predisposition are not automatically predisposed to develop severe periodontitis. Although direct data are not yet available, one would expect that removing the bacteria would ameliorate the effect of a risk factor such as the CD14 genotype. In the present study, however, 58.0% of the healthy subjects were also positive for this genotype.
Additionally, a novel mutation in the promoter of the CD14 gene was identified in a heterozygous combination. The functional significance of this deletion is unclear at present. Computational search for the transcription factors binding site of the CD14 gene promoter showed no transcriptional factor binding to this region.
In conclusion, the CD14 –1359G/T polymorphism was observed in association with the severity of chronic periodontitis. In recent years, it has become evident that for many common chronic diseases, there are modifying factors that do not cause the disease but rather amplify some disease mechanisms to make the clinical condition more severe.31 Several studies have suggested there is a substantial genetic influence in periodontal disease. In recent years, for example, interleukin-1,22,32–34 interleukin-4,35 and immunoglobulin G Fc receptor gene36 polymorphisms have been associated with an increased risk of chronic or aggressive periodontitis. To our knowledge, the present study is the first one documenting evidence of the role of the CD14 genotype as a risk factor in chronic periodontitis. More extensive studies in larger groups of patients, and also in other ethnic populations, should be undertaken in order to analyse the putative relevance of the CD14 –1359G/T (−159C/T) polymorphisms in the pathogenesis of periodontitis. We conclude that these analyses need to be repeated in a larger sample size before definitive conclusions can be drawn.
Acknowledgments
The study was carried out as a part of the project CEZ 307/98:141100002 and project FRV No 613, supported by the Ministry of Education, Youth and Physical Training of the Czech Republic. We would like to thank Andrea Stejskalova for excellent technical assistance.