Article Text
Abstract
Hereditary haemorrhagic telangiectasia (HHT) is a genetic vascular disorder characterised by epistaxis, telangiectases, and visceral manifestations. The two known disease types, HHT1 and HHT2, are caused by mutations in the endoglin (ENG) and ALK-1 genes, respectively. A higher frequency of pulmonary arteriovenous malformations (AVMs) has been reported for HHT1 while HHT2 is thought to be associated with a lower penetrance and milder disease manifestations. In this study, we present 10 families with an ALK-1 genotype. Visceral manifestations were detected in 24 (26%) of the 93 HHT2 patients from nine of the families and included gastrointestinal bleeding (14%), intrahepatic shunts (6%), and AVMs in the lung (4%) and brain (3%). Gastrointestinal bleeding, the most frequent visceral manifestation, was reported in six of the 10 families, mostly in patients over the age of 50. These patients also had frequent epistaxis and suffered from anaemia, often requiring blood transfusions. The identification of ALK-1 mutations in subjects with a suspected diagnosis and without clinical signs of HHT argue in favour of a molecular diagnosis. We also analysed the data published on 44 families with HHT2 and conclude that visceral manifestations occur in 26 of these families and affect 30% of HHT2 patients. This is considered an underestimate given incomplete and variable screening for lung, brain, and/or liver involvement in different clinical centres. These findings, however, stress the need for an early diagnosis of HHT that can be useful for the early control of associated visceral involvement.
- vascular disorder
- TGF-β receptors
- arteriovenous
- visceral manifestations
- HHT, hereditary haemorrhagic telangiectasia
- CT, computed tomography
- CE, contrast echocardiography
- MRI, magnetic resonance imaging
- MRA, magnetic resonance angiography
- AVM, arteriovenous malformation
Statistics from Altmetric.com
- HHT, hereditary haemorrhagic telangiectasia
- CT, computed tomography
- CE, contrast echocardiography
- MRI, magnetic resonance imaging
- MRA, magnetic resonance angiography
- AVM, arteriovenous malformation
Hereditary haemorrhagic telangiectasia (HHT) is an autosomal dominant vascular disorder with an incidence greater than 1/10 000.1–4 The most common presenting sign is epistaxis, reported in 78–96% of cases.1,5–8 Telangiectases are frequently observed, predominantly on the nasal and oral mucosa and the skin of the face and hands.1,9,10
Significant proportions of patients with HHT have pulmonary and cerebral vascular malformations. A pulmonary arteriovenous malformation (AVM) consists of a direct communication between a pulmonary artery and vein resulting in a low resistance right to left shunt. Pulmonary AVMs have been reported in 5–50% of HHT patients1,9,11 and can lead to serious complications. These include stroke (30–40%), cerebral abscess (5–20%), or massive pulmonary haemorrhage owing to spontaneous rupture of a pulmonary AVM.12–18 Cerebral AVMs occur in 5–20% of adults and children and can lead to seizures and life threatening or disabling haemorrhagic stroke.9,19–21 Gastrointestinal (GI) bleeding is estimated to be present in 13–44% of affected subjects, usually in the fifth and sixth decades.1,5,9,22,23 GI bleeding is predominantly the result of telangiectases in the stomach and small bowel, though other parts of the GI tract may also be involved.22 GI bleeding and epistaxis often lead to anaemia with low serum iron, low ferritin, and high transferrin levels.22,24
Hepatic involvement now appears to be more common (up to 32%)25–27 than previously thought1,28 and is the result of the presence of multiple intrahepatic telangiectases. Advanced stages of disease can be associated with cirrhosis and liver dysfunction but more commonly the left to right shunting leads to high cardiac output and heart failure.25,29–31
HHT is a genetically heterogeneous disorder as mutations in two genes, endoglin (ENG) and ALK-1 (ACVRL1), are the cause of HHT1 and HHT2, respectively.3,32–34 This genetic heterogeneity is also reflected in the clinical variability between the two types of HHT. Thus, pulmonary AVMs were reported to be more frequent in HHT1, while lower penetrance, milder phenotype, and a later onset of disease are thought to be associated with HHT2.3,10,35–39 An increased prevalence of liver involvement has recently been suggested for families with mutations in the ALK-1 gene.40–42 However, the phenotype of families with the HHT2 genotype remains poorly understood owing to the small number characterised to date.
In this study, we report clinical data on 10 families with known mutations in the ALK-1 gene. We also summarise the published data on patients and/or families with a known HHT2 genotype.
MATERIALS AND METHODS
Patients, families, and samples
Members of 10 families were recruited from HHT clinical centres in Europe and North America. The clinical diagnosis of HHT was made according to the Curaçao criteria43 and included the presence of three of the four features: a family history of HHT, spontaneous recurrent epistaxis, telangiectases, and visceral involvement such as gastrointestinal bleeding, pulmonary and cerebral/spinal AVMs, or intrahepatic shunting. The presence of two criteria is recorded as a possible or suspected HHT, while fewer than two criteria are considered as an unlikely HHT.43 Subjects with the familial ALK-1 mutation (mostly younger adults and children), irrespective of current clinical status, were also considered to have HHT2 and included in the analysis.
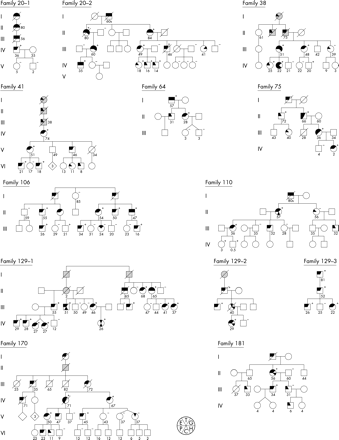
The pedigrees were prepared from medical records and interviews with family members and are presented in fig 1. Screening of patients for visceral involvement was performed according to the centre specific protocols.
Pedigrees of the 10 HHT2 families. Numbers under the symbols indicate the age of the patients (years) at the time of manuscript preparation or at time of death. Each symbol is divided into six sections depicted in the figure. Each section represents one of the following: epistaxis (E), telangiectases (T), GI bleeding (G), hepatic shunt (H), cerebral/spinal AVM (C), or pulmonary AVM (P). Grey symbols represent uncertain clinical status or manifestations, but suspected of HHT based on reports of recurrent nosebleeds and with children and/or parents with confirmed HHT. + and − represent, respectively, the presence or absence of a familial germline ALK-1 mutation as determined by sequencing.
Screening for visceral manifestations of HHT
Screening protocols varied by centre; however, all centres (5/5) routinely offered screening for PAVMs and 4/5 centres routinely offered screening for CAVMs. In all centres, PAVM screening protocols for adults were sensitive protocols (agitated saline transthoracic echocardiography, oxygen shunt test, unenhanced helical computed tomography (CT), or magnetic resonance angiography (MRA)). For children, sensitive PAVM screening protocols (contrast echocardiography (CE) or unenhanced helical CT) were used in 2/5 centres when feasible without sedation, and a less invasive and less sensitive “basic” protocol (chest radiography and/or pulse oximetry) for the remaining children as well as in the other three centres. Patients with suspected PAVMs based on screening were offered diagnostic pulmonary angiography and endovascular treatment. CAVM screening was performed with cerebral MRI (magnetic resonance imaging) in all centres, and rarely with cerebral CT in patients with contraindications to MRI or when MRI was unavailable. Patients with suspected CAVMs based on screening were offered diagnostic cerebral angiography and endovascular or surgical treatment. Hepatic screening protocol was basic and not sensitive in 2/5 centres (physical examination for hepatic bruit and/or serum liver function tests) and sensitive in 3/5 centres (mesenteric Doppler, enhanced helical CT, or MRI). Patients with a suspected hepatic shunt based on screening were offered MRA or diagnostic mesenteric angiography if symptomatic. Basic screening for GI telangiectasia included only testing for iron deficiency anaemia. Patients with suspected GI bleeding based on screening were offered diagnostic endoscopy in all centres.
Mutation analysis
Genomic DNA was prepared from peripheral blood lymphocytes of patients and from placenta and human umbilical vein endothelial cells (HUVEC) from newborns using Puregene™ DNA Isolation Kit (Gentra Systems). The procedures were reviewed and approved by the Research Ethics Board of the Research Institute of the Hospital for Sick Children, Toronto, Canada.
All nine coding exons of ALK-1 were analysed by sequencing using the Open Gene Automated DNA Sequencing System II (VGI) as described previously.44 The identified ALK-1 mutations44,45 are presented in table 1.
Summary of ALK-1 mutations in 10 HHT2 families
RESULTS
A total of 234 subjects in 10 families were traced (fig 1). Of these, 33 were unaffected spouses and thus excluded from the study. Seventy-five subjects were found to have a definite diagnosis of HHT based on the clinical criteria. Another nine were considered to have HHT as they were dead parents of confirmed HHT patients and had a known history of recurrent nosebleeds. Twenty subjects were suspected of having HHT as defined by the presence of only two criteria (telangiectases or epistaxis and a positive family history). Fifteen of these were excluded from the analysis since five did not carry the familial ALK-1 mutation and another 10 could not be resolved because of the unavailability of their DNA samples. The remaining five suspected cases carried the respective familial ALK-1 mutations. Another four subjects (aged 21, 20, 3, and 0.5 years) with disease associated mutations currently had no clinical manifestations. All of these subjects (n=93) were considered HHT2 positive and included in the final analysis of clinical manifestations (tables 2 and 3). There were 51 males (range 0.5–81 years, mean 39.8) and 42 females (range 2–80 years, mean 45.3).
Screening for visceral involvement in 93 HHT2 patients
Visceral manifestations in reported HHT2 families
Screening of HHT2 patients for visceral involvement
Of the 93 HHT2 patients, as defined by clinical criteria and/or presence of an ALK-1 mutation, the following were screened for visceral involvement as indicated in table 2. Forty patients (43%) underwent screening for pulmonary AVMs. Sensitive screening protocols (contrast echocardiography, oxygen shunt test, unenhanced helical CT, or magnetic resonance angiography) were used in 38 of these patients and two patients were screened by chest radiography alone (table 2). CAVM screening was performed in 27 (29%) of the 93 patients. Cerebral MRI was the method of choice in all except one patient (CT). Spinal AVMs were also detected in two symptomatic patients using spinal MRI.
Screening for GI involvement was based on the presence of iron deficiency anaemia in 24 patients with GI bleeding (26%), despite iron replacement and treatment of epistaxis, or despite only mild or no epistaxis (table 2). GI telangiectases were detected by endoscopy in four of these patients and observed in another patient during unrelated GI surgery. Basic screening was used to screen for hepatic shunts in 24 patients (26%) (table 2). Nine of these (10%) were also screened by mesenteric Doppler and six (6%) symptomatic patients underwent mesenteric angiography.
Family 20
The missense mutation identified in this family is a G1232 to A substitution that leads to conversion of Arg411 to Gln45 (table 1). This mutation was also previously reported in an unrelated family with HHT.41 In family 20-1, only one of the four known HHT2 patients was alive at the time of study (fig 1). The proband, a 36 year old man, started exhibiting external telangiectases on his face, upper back, nasal mucosa, and mouth, as well as daily epistaxis, in his early thirties. His father was found to have internal telangiectases that caused severe bleeding during unrelated gastrointestinal surgery. The proband’s affected grandmother and great grandmother also had a history of GI bleeding. The proband and his father were screened by chest radiography. None was screened for brain involvement. His two young children, aged 5 and 2, did not carry the familial ALK-1 mutation.
Family 20-2 was suspected to be distantly related to family 20-1 following mutation identification. Both are North American families with European ancestors of German and French origins, respectively. A partial pedigree was previously reported and mutation analysis carried out on a number of family members.38 The dead ancestor in generation I and two of his daughters were clinically affected and suffered from GI bleeding. The dead 80 year old woman had frequent GI bleeding that started at the age of 69. The bleeding became increasingly severe and required iron replacement therapy and blood transfusions of 4–5 units every 10 days. At the age of 78, she had undergone oestrogen-progesterone treatment that ended the GI bleeding and transfusion needs within a month and reduced her nosebleeds. Her 60 year old daughter had recurrent spontaneous epistaxis and infrequent GI bleeding since the age of 55. Screening by contrast echocardiography and brain MRI showed no pulmonary or cerebral AVMs but mesenteric Doppler sonography and functional tests were suggestive of liver involvement (table 2). The 35 year old grandson had numerous typical telangiectases and recurrent spontaneous epistaxis. He also had a history of GI bleeding since the age of 32. Screening for pulmonary and cerebral AVMs and intrahepatic shunting was negative (table 2).
The 84 year old woman of generation II also had a history of GI bleeding. She had approximately 20 episodes since their onset at the age of 65, all requiring blood transfusions. Her 49 year old daughter and her three children, who had epistaxis of varying frequency but no known visceral manifestations, all carried the familial ALK-1 mutation. The 46 year old son was not screened for visceral involvement and died of an unrelated cause.
Family 38
A deletion of G at position 86 was identified in five members of this North American family45 (table 1). It leads to a frameshift and termination at codon 31.
Two brothers in generation II had HHT. The first one had a history of severe nosebleeds, long standing anaemia, and was “found dead in a pool of blood”. The second brother was disabled as a result of a stroke at the age of 48. No cerebral AVM was detected on screening and the stroke was probably the result of a pulmonary AVM for which he was never screened. He had a number of transient ischaemic attacks and/or small strokes before a fatal stroke at the age of 75. His 51 year old daughter had a history of GI bleeding since the age of 43. She also had migraines and chronic anaemia, suspected to be because of severe epistaxis and requiring iron infusions and blood transfusions. No pulmonary or cerebral AVMs were detected by CT or MRI/MRA but an abdominal bruit was heard on physical examination and intrahepatic shunting was confirmed by ultrasound (table 2). Mutation analysis confirmed that she was affected as were her two daughters. The older one, aged 25, had infrequent epistaxis (3–6 per year) but was not screened for visceral involvement. The 22 year old daughter had recurrent epistaxis since the age of 6 and a long history of migraines. A single complex pulmonary AVM and a right posterior cerebral artery AVM were detected by routine screening (CT, MRI, examination for bruit) at the age of 17. The pulmonary AVM was successfully treated with transcatheter embolisation and the cerebral AVM excised following preoperative embolisation. By the age of 21, epistaxis and recurring bleeding from a telangiectasia on her tongue had become a significant problem and laser coagulation treatment was scheduled. The 21 year old son had no clinical manifestations and did not carry the ALK-1 mutation.
The 48 year old woman of generation III had moderate epistaxis but routine screening (CT, MRI, examination for bruit) showed no visceral involvement (table 2). An ALK-1 mutation was also found in her 20 year old son. However, he exhibited no disease manifestations and careful physical examination, medical history, and screening for visceral involvement were negative. His sister, aged 22, did not have clinical signs of the disease, nor the family mutation. The 39 year old woman of generation III who had rare epistaxis (less than once a year) was found not to carry the familial mutation.
Family 41
In this American family, the disease associated ALK-1 mutation is a G998 to T substitution that leads to a change of Ser333 to Ile45 (table 1). This mutation was found in four of the five subjects tested and correlated with the presence of epistaxis and telangiectasia. This family is probably derived from the same ancestor as the large Utah family previously described.47
No definite clinical information was available for members of generations I-II. The subject in generation III had epistaxis and anaemia and died of an unrelated cause at the age of 38. In generations IV-VI, screening (chest CT, brain MRI/MRA) of the five subjects with a clinical diagnosis of HHT did not show pulmonary or cerebral AVMs (table 2). Medical history and physical examination (listening for an abdominal bruit) showed no hepatic involvement. The 74 year old woman from generation IV had severe epistaxis since childhood and had undergone multiple cauterisations and septal dermoplasty. Her 51 year old daughter had mild epistaxis, first appearing at the age of 26. Two of her three children also had epistaxis of varying severity and were found to carry the familial mutation.
The 46 year old man of generation V had telangiectasia and epistaxis (3–4 per week) but no detectable pulmonary or cerebral AVMs on screening (table 2). His 11 year old daughter suffered from nosebleeds (three to four per month) since the age of 9 months while his 8 year old son had nosebleeds (two to three per month) since the age of 6 years. The children did not undergo medical evaluation or screening and did not have their DNA tested.
Family 64
The ALK-1 mutation found in this English Canadian family is a G1221 to T substitution that converts Glu407 to Asp44 (table 1). It was confirmed in two clinically affected patients.
In addition to regular epistaxis, the 57 year old grandfather had a history of severe GI bleeding complicated by anaemia and requiring blood transfusions. Both of his children had recurrent spontaneous epistaxis. Mutation analysis confirmed HHT2 in the daughter, aged 28, and no pulmonary or cerebral AVMs were detected upon screening with sensitive protocols (table 2). Her twin children, whose DNA samples were tested shortly after birth, did not carry the familial mutation. The 31 year old man of generation II had a history of epistaxis but declined clinical and molecular screening.
Family 75
A T1123 to C substitution in exon 8 of ALK-1 was found in four members of this American family45 (table 1). This missense mutation converts Tyr375 into His. Five of the 12 subjects in this family were clinically affected. In generation II, the 72 year old man and his daughter had epistaxis only. Mutation analysis supported an HHT diagnosis in the father but the daughter was not tested molecularly.
His 66 year old brother had a pulmonary AVM (CE, unenhanced helical CT) (table 2). The familial mutation was found in the 36 year old daughter who also had a pulmonary AVM, but not in the asymptomatic 4 year old grandson. The 2 year old granddaughter, who already had occasional epistaxis (more than once a month), also carried the familial mutation.
Family 106
A frameshift mutation caused by an insertion of G at position 1113 was found in the 54 year old proband and confirmed in another seven family members45 (table 1). This family originated from the Rhone-Alpes region where the prevalence of HHT is the highest in France.3 Ten family members had a clinical diagnosis of HHT. In generation II, five of the seven subjects had HHT and suffered from anaemia. One patient, aged 47, had a single episode of GI bleeding which was treated. No pulmonary AVM was detected on screening by CT (table 2). His 16 year old son had three telangiectases on the lips and fingers, and nosebleeds since the age of 4 years. An ALK-1 mutation was also found in three clinically affected subjects (aged 26, 34, and 16) of generation III. The fourth subject, a 21 year old man, did not exhibit HHT manifestations but carried the familial mutation. This is probably a case of non-penetrance and he may still develop clinical manifestations later in life. His 24 year old cousin who had a single telangiectasia did not carry the mutation.
Family 110
The missense mutation found in this English Canadian family has previously been reported.44 It is a G150 to T substitution that leads to a change of Trp50 to Cys (table 1). This four generation family had four clinically affected adults (fig 1). The now dead ancestor had severe GI bleeding since his 20s. He also had facial telangiectases, daily epistaxis, anaemia, and required blood transfusions. He died in his 80s of heart failure. His 57 year old daughter had intermittent severe bleeding from telangiectases on her tongue, anaemia, and required blood transfusions. She had a small cerebral AVM that had spontaneously thrombosed after diagnostic angiography (table 2). There was no upper GI bleeding but she had one episode of lower GI bleeding of unknown cause. Screening for liver involvement was inconclusive as she had only a mildly increased gamma-glutamyl transpeptidase (GGT) and otherwise normal functional tests. Her mesenteric Doppler showed a mild increase in celiac blood flow, but insufficient for diagnosis of intrahepatic shunt owing to liver telangiectases. Her two daughters, one clinically affected, were both screened (CE, chest radiography, brain MRI) and no pulmonary or cerebral AVMs were detected (table 2). Two of the grandchildren, aged 3 years and 6 months, currently exhibit no signs of HHT but carry the familial ALK-1 mutation. The 32 year old man of generation III had infrequent epistaxis and two telangiectases, but was not screened for other organ involvement.
The 56 year old woman of generation II had a history of severe epistaxis in childhood and had undergone cauterisation at the age of 9. Nosebleeds have since subsided and she did not carry the familial ALK-1 mutation, indicating that the childhood nosebleeds were not HHT related. Her younger son, aged 32, also had epistaxis as a child.
Family 129
Three families originating from the same area of “Parthenay, Département des Deux Sévres” (western France) were shown to carry the same ALK-1 mutation. It is a G1121 to A mutation that leads to substitution of Arg 374 for Gln45 (table 1). Families 129-1 and 129-2 were found to share an ancestor and it is likely that family 129-3 also derives from the same common founder.
Family 129-1 included 17 clinically affected subjects (fig 1). Two patients from generation II suffered from GI bleeding owing to gastric telangiectases and complicated by chronic anaemia. Another, a 51 year old man from generation III, had a sudden paraplegia at the age of 22 owing to a spinal AVM that was not embolised at the time. He is currently wheelchair bound and has epistaxis. His 26 year old niece was first seen at the age of 2 years for a sudden paraparesis owing to compression by a large intradural extramedullary spinal AVM. At that time, embolisation was not performed. Neurological symptoms worsened by the age of 16 and embolisation of the AVM was performed. She is currently wheelchair bound with bladder and sphincter dysfunction. She has no nosebleeds and only one telangiectatic lesion on her tongue. Another four affected subjects were screened by chest CT and no pulmonary AVMs were detected (table 2).
Family 129-2 included five HHT2 patients. The man in generation II and one of his sons were clinically affected and carried the familial ALK-1 exon 8 mutation. His daughter died at the age of 40 of complications associated with liver AVM before a liver transplant could be performed. His 29 year old granddaughter also had a liver AVM. She had undergone liver transplantation at the age of 26, after which her daily epistaxis stopped. Brain MRI and lung CT scan detected no cerebral or pulmonary AVMs (table 2). She is currently pregnant and the fetus is healthy and free of HHT.
In family 129-3, four subjects with HHT2 had mucocutaneous telangiectases and nosebleeds. The onset of epistaxis was at 43 years in the 52 year old man and at 13 years in his 26 year old son. The second son, aged 25, was not clinically affected and did not carry an ALK-1 mutation. The 22 year old daughter had epistaxis and one telangiectasia on the wrist and two on the tongue. Mutation analysis confirmed that she carried the familial mutation.
Family 170
This is a large German family spanning six generations (fig 1). A missense mutation, C1231 to T, that changes Arg411 to Trp, was found in the 71 year old female proband45 (table 1) of generation IV. She had a pulmonary AVM (positive echobubble and 89% SaO2) (table 2) and died in hospital from pneumonia. Her mother died at the age of 72 after severe and progressive episodes of epistaxis. Two of her children and a grandson were also clinically affected. Another grandson, aged 11, was suspected of having HHT as he had nosebleeds only. Her 37 year old son had one episode of epistaxis a year and did not carry an ALK-1 mutation.
The proband’s 67 year old sister and her 45 year old son had HHT. Both were screened for pulmonary AVMs and none was detected (table 2). The two grandchildren, aged 15 and 13, were not affected. Her three daughters, one with a single telangiectatic lesion (and consequently their young children), did not carry the familial mutation. The proband’s affected cousin died at the age of 71 of an unknown cause. He was not screened for pulmonary and cerebral involvement but had epistaxis and telangiectases. His father, who also had epistaxis and telangiectases, had died in an accident at the age of 35. The intensity of epistaxis in all the affected patients was moderate with onset ranging from 14 to 40.
Family 181
A frameshift mutation was found in this English Canadian family. It results from a deletion of C1299 in exon 9 of the ALK-1 gene and leads to a frameshift at Pro 433 and truncation at codon 43845 (table 1). In this family, three patients had a clinical diagnosis of HHT and another three were suspected of having the disease as they had only epistaxis (fig 1). The dead patient of the first generation had severe epistaxis requiring blood transfusions and numerous nasal embolisations. No cerebral AVM was detected by angiography (table 2). However, screening for pulmonary AVM was not performed and he died of congestive heart failure. His older son, aged 65, was asymptomatic while the 33 year old grandson had frequent epistaxis. However, they were not screened clinically or tested molecularly.
The 56 year old woman of generation II had telangiectatic lesions and recurrent spontaneous epistaxis. Screening for pulmonary (chest radiography, CE) or cerebral AVMs (MRI) was negative (table 2). Serum liver function tests, however, indicated possible liver involvement that was confirmed by mesenteric Doppler and MRI/MRA (table 2). Her 34 year old son had telangiectases and epistaxis (once a month) but no pulmonary or cerebral AVMs detected on screening (table 2). The 4 year old twin granddaughters currently exhibit no disease manifestations. The second son, aged 31, had epistaxis (once a week) but did not carry the familial ALK-1 mutation. The 6 year old son also had nosebleeds but was not screened or tested molecularly. Both brothers have been screened and no cerebral or pulmonary AVMs detected.
DISCUSSION
We report the clinical findings in 10 families with a molecular diagnosis of HHT2. Visceral involvement was present in members from nine of the 10 families. Screening led to the detection of pulmonary AVMs in four patients from three families while MRI showed cerebral AVMs in two patients from different families and spinal AVMs in two relatives from another family. The most frequent manifestation observed was symptomatic GI bleeding, found in 14 patients from six families. The liver was involved in six patients from four families. In total, 24 (26%) of the 93 HHT2 patients, as defined by clinical criteria and/or the presence of an ALK-1 mutation, had at least one visceral manifestation. Four patients had two affected organs (three GI and liver and one lung and brain).
If one includes all the 44 documented families with mutations in the ALK-1 gene34,3840–42,44,45,47,48 (table 3), 27 families (61%) had visceral involvement. A total of 82 cases were documented in the 281 patients with HHT2, indicating that up to 29% of patients with HHT2 had developed visceral manifestations (table 3). In this group of patients, hepatic involvement was observed in 13%, followed by gastrointestinal bleeding in 12%, pulmonary AVMs in 5%, and cerebral/spinal AVMs in 3%. However, one should take into consideration the variation in screening protocols and the extent of screening in the different centres, particularly for hepatic involvement and cerebral AVMs. Furthermore, the age of patients is important as some manifestations, such as GI bleeding, generally occur later in life. The given incidence of observed visceral manifestations (29%) should therefore be considered an underestimate in HHT2 patients.
Patients with HHT are known to be at risk of developing visceral telangiectases or AVMs. However, the majority of published studies looked at the clinical manifestations of HHT, irrespective of genotype, and concluded that pulmonary AVMs were present in more than 30% and cerebral AVMs in 5–20% of patients.1,9,11,18,20,49 Several studies have suggested that pulmonary AVMs are more frequent in HHT1 than HHT2 patients and that cerebral AVMs are often observed in families with pulmonary AVMs.35–39 In a report describing families with mutations in the ENG gene, pulmonary AVMs were found in 21 and cerebral AVMs in 11 of the 24 families.50 There are also several case reports of newborns and children with cerebral AVMs. A recent paper described nine infants and children who presented with intracranial haemorrhage secondary to cerebral AVMs: six had HHT1 and the remaining three had an unknown HHT genotype.21 The data summarised in table 3 suggests that the prevalence of pulmonary AVMs in HHT2 (5%) is lower than reported for HHT1. Cerebral and spinal AVMs were found in 3% of patients with HHT2, which is also lower than reported for patients with HHT, implying that the incidence is higher in HHT1.
Gastrointestinal bleeding was previously reported to occur in 20–30% of HHT patients.1,22 The incidence of GI bleeding in all reported HHT2 patients is 12% (table 3). In the analysis of 38 patients from a large Utah family with HHT2,47 four cases of GI bleeding were documented as well as hepatic shunts (n=5), pulmonary (n=2), cerebral (n=2), and spinal (n=1) AVMs. In another study of a Taiwanese family, GI bleeding and severe hepatic involvement were detected in three of the five subjects carrying an ALK-1 mutation.41 The other two subjects (1 and 5 years old, respectively) were too young to manifest HHT symptoms. GI manifestations were also reported in five of 12 (42%) subjects from a large Danish family and in only one (5.8%) patient in a second family.48 However, since GI bleeding generally occurs in the fifth or sixth decade of life, its incidence in HHT2 is likely to increase with age.
Liver involvement in HHT has been recognised by instrumental screening in up to 32% of patients25,29,51 and is usually asymptomatic.25 Clinical manifestations of liver involvement in patients with HHT include heart failure, portal hypertension, biliary disease, encephalopathy, and abdominal pain.25,29,31,51 These manifestations are reported mostly in later stages of the disease and depend on the type and size of the shunt as well as on the effects of an abnormal hepatic blood supply.25,31,52
A high incidence of liver involvement was reported in several studies of patients with ALK-1 mutations.40–42,47 In all published HHT2 families, liver involvement was found in 36 patients (13%) (table 3). However, it is difficult to estimate the exact occurrence of liver involvement as most centres do not routinely perform liver screening and it is likely to be undiagnosed in a substantial number of patients. To date, treatment has been limited to symptomatic care by embolisation, ligature of the hepatic artery, or liver transplantation, all performed with different degrees of success.53,54 This further contributes to the controversy over the need and extent of screening for liver involvement in HHT patients.
High phenotypic variability within HHT2 families makes it difficult to assess any potential correlation between clinical symptoms, position, or type of an ALK-1 mutation. We have previously reported that the majority of ALK-1 mutations were found in exons 3, 4, 7, and 8 which encode for the extracellular, transmembrane, and kinase domains respectively.45 The publication of additional 13 ALK-1 mutations42 brings the total number to 50 with 36% in exons 3 and 4 and 23% in exons 7 and 8. However, only exons 3, 7, and 8 were sequenced in the study of Olivieri et al,42 which might now overestimate the frequency of mutations in these exons.
Missense mutations found in the kinase domain occur at residues that are conserved among all type I receptors of the TGF-β superfamily.45 Alterations in polarity, charge, hydrophobicity, and/or size of the substituted amino acid caused by these mutations will likely have structural effects creating misfolded proteins.45 Mutations causing a frameshift will yield unstable transcripts and/or truncated proteins. Thus, most ALK-1 mutations probably lead to non-functional proteins, although one cannot rule out the possibility that some mutants might be expressed and could interfere with the normal function of ALK-1.
Limited phenotypic description has been reported for HHT2 families. Visceral manifestations were described in 17/24 families with ALK-1 missense mutations, of which 12 had members with GI bleeding and/or hepatic shunts. Out of another 10 families with frameshift mutations (five deletions and four insertions), five showed visceral manifestations with hepatic shunts in members from three families. In another five families with nonsense mutations (n=5), hepatic shunts were present in two families. These data suggest that hepatic involvement and/or GI bleeding occur both in families with missense or truncation mutations. Large numbers of additional mutations in families with complete phenotypic analysis are needed to estimate any potential correlations between mutation type and severity of disease.
It should be mentioned that mutations in the ALK-1 gene have been identified in patients with primary pulmonary hypertension (PPH).46 Mutations in BMPRII, a type II receptor belonging to the TGF-β superfamily, generally lead to familial or sporadic forms of PPH.55,56 Since PPH is a very rare disease estimated at 1 in 2 million,57 it has not been readily observed in HHT families. However, several HHT2 families were identified among the cohort of pulmonary hypertension patients.46ALK-1 mutations associated with PPH are also of varying types and found in different exons. These data suggest that a reduction in the level of ALK-1 can cause an imbalance in the regulation of TGF-β/BMP mediated endothelial pathways, ultimately leading to HHT and, in a small percentage of patients, to PPH.
Our data indicate that 60% of HHT2 families reported include members with at least one visceral manifestation and that 30% of all patients have either pulmonary, cerebral, hepatic, or gastrointestinal complications. This number is an underestimate in HHT2 patients and is likely to increase in centres implementing screening for visceral involvement with sensitive protocols. As clinical manifestations do not often occur until later in life, the development and implementation of a molecular diagnosis will allow the identification of subjects with no evident signs of the disease but carrying the familial mutation. This becomes important in order to implement suitable screening protocols with an objective to control the local and systemic symptoms of the disease and to prevent complications. Furthermore, it will allow the determination of family members who do not carry the disease associated mutation, and therefore do not need to undergo further clinical screening.
Acknowledgments
This research was supported by grant HHT-FY02-226 from the March of Dimes and by grant T-5016 from the Heart and Stroke Foundation of Canada. We are grateful to all patients and family members who participated in our studies. We thank all the physicians and genetic counsellors who provided clinical information.