Abstract
Noonan syndrome (NS) is a relatively common, but genetically heterogeneous autosomal dominant malformation syndrome. Characteristic features are proportionate short stature, dysmorphic face, and congenital heart defects. Only recently, a gene involved in NS could be identified. It encodes the non-receptor protein tyrosine phosphatase SHP-2, which is an important molecule in several intracellular signal transduction pathways that control diverse developmental processes, most importantly cardiac semilunar valvulogenesis. We have screened this gene for mutations in 96 familial and sporadic, well-characterised NS patients and identified 15 different missense mutations in a total of 32 patients (33%), including 23 index patients. Most changes clustered in one exon which encodes parts of the N-SH2 domain. Five of the mutations were recurrent. Interestingly, no mutations in the PTPN11 gene were detected in five additional patients with cardio-facio-cutaneous (CFC) syndrome, which shows clinical similarities to NS.
Similar content being viewed by others

Introduction
Noonan syndrome (NS), MIM 163950, is a well-known developmental disorder. Its prevalence has been estimated as 1 in 1000–2500 live births. Familial cases are consistent with autosomal dominant inheritance with marked phenotypic variability, but most of the cases appear to be sporadic and may represent new mutations. The main features of NS include a typical facial appearance, congenital heart defects, and short stature. Developmental delay is frequent, and mild mental retardation is relatively common. Other frequent manifestations include pectus deformities and cryptorchidism in males.
Using linkage analysis in several families, a gene for NS was first mapped to a 14 cM segment of chromosome 12q24.1 Interestingly, one of the families did not show linkage to this region thereby providing evidence for genetic heterogeneity. Subsequently, Legius et al2 narrowed the interval to a 5 cM region between markers D12S84 and D12S1341. By testing genes of this chromosomal region, Tartaglia et al3 recently identified a candidate gene, PTPN11, which encodes the non-receptor protein tyrosine phosphatase SHP-2. Mutation analysis in two families and 22 sporadic cases revealed a number of missense mutations. More recently, studying a larger patient set, PTPN11 mutations were detected in 45% of unrelated individuals with sporadic or familial NS.4
In this study, we present the results of the mutation analysis of the PTPN11 gene in 96 well-characterised patients with NS, including 79 index patients and 17 relatives. Furthermore, our study suggests that mutations in PTPN11 are not a frequent cause of the clinically overlapping CFC syndrome.
Materials and methods
Patients' sample collection
All patients and their parents were seen by experienced clinical geneticists or in the department of cardiology by HG Kehl, and most of them were evaluated following a standard protocol, which consisted of an interview, a detailed clinical examination, measurements of growth parameters, and echocardiography. A diagnosis of NS was made when suggestive facial features and one additional feature such as characteristic heart defect, growth retardation, cryptorchidism, mild mental retardation, or pectus deformity were present. In patients with less characteristic facies a family history of NS or two additional features were required.5 After informed consent, blood samples were collected, and DNA was extracted using standard protocols.
Mutation screening
For mutation analysis of the PTPN11 gene, we amplified all 15 coding exons from DNA of the patients using primers located in flanking introns. PCR reactions were carried out in 100 μl reaction volumes containing 100 ng of genomic DNA, 30 pmol of each primer, 0.4 mM dNTPs, 0.8 U Amplitaq DNA polymerase (Applied Biosystems), 3 mM MgCl2. For exons 4 and 8, amplifications were performed in the presence of 4 mM and 3.5 mM MgCl2, respectively. Cycling conditions comprised an initial denaturation step at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at the specific annealing temperature of the primers for 30 s, extension at 72°C for 45 s, and a final extension step at 72°C for 10 min. Amplification of an appropriately sized PCR product was confirmed on 1.5% agarose gels. Primer sequences are available upon request.
For DHPLC, an aliquot of each PCR product was analysed using the WAVE nucleic acid fragment analysis system (Transgenomic). Melting profiles and resolution temperatures (Tr) were predicted by the Transgenomic WAVEMAKER™ software version 4.1. Immediately prior to DHPLC analysis, PCR products were denatured at 95°C for 5 min and then gradually cooled to 25°C over a period of 30 min. Re-annealed DNA duplexes (8 μl) were injected and eluted with a linear acetonitrile gradient at a flow rate of 0.9 ml/min, with a mobile phase consisting of a mixture of buffer A, 0.1 M triethylammonium acetate (TEAA) with 0.1% acetonitrile and buffer B 0.1 M TEAA with 25% acetonitrile. The remaining aliquot of PCR products that showed an altered elution profile and a new PCR amplified product were purified with the QIAquick gel extraction kit (Qiagen), sequenced with the BigDye Terminator Chemistry (PE Biosystems) and separated on an ABI 377 DNA sequencer.
Results
The genomic organisation of the human PTPN11 gene was established by comparing the cDNA (GenBank Accession NM_002834) with the genomic sequence of the clones RPC1-66E7 and RP3-329E11 (GenBank Accessions AC004216, AC004086). All 15 coding exons and flanking intronic sequences were screened for the presence of mutations. Mutation analysis was carried out by denaturing high performance liquid chromatography (DHPLC), followed by bidirectional sequencing of all samples showing abnormal chromatograms suggestive of mismatches in the heteroduplex DNA.
The patient set comprised 11 families, with 28 family members without linkage data and 68 apparently unrelated sporadic cases. We detected a mutation in five index patients and nine of their relatives, and in 18 sporadic cases. In total, we identified 15 different missense mutations in 32 subjects, i.e. in 29% of the 23 index patients, but no frameshift or stop mutations (Table 1). Eleven different mutations were only present in sporadic and not in familial cases. In all five families the mutation co-segregated with the disease. None of the changes were present in 95 control subjects of the same ethnic origin. The clinical data of patients with a mutation are summarised in Tables 2 and 3.
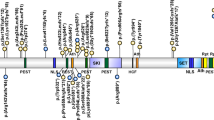
The majority of mutational events (73%, 11 out of 15) involved amino acids located in exon 3, which is part of the N-SH2 domain (Figure 1). Twenty per cent (three out of 15) of the mutated amino acids belong to the PTP domain. Only one mutation was detected in the C-SH2 domain (417G→C) leading to a substitution of glutamic acid in position 139 by aspartic acid. Four mutations were found more than once, three of which, i.e. the A182G, A188G and G214A are located in exon 3, whereas the A922G change lies in exon 8 (Figure 1). Five mutations identified in our study have not been described before3,4, including 174C→G (Asn58Lys), 205G→C (Glu69Gln), 211T→C (Phe71Leu) and 214G→A (Ala72Ser), which are located in the N-SH2 domain, and 767A→G (Gln256Arg), which lies in the PTP domain. The most common mutations are A188G, detected in four index patients and five relatives, and A922G which was present in four index patients and one relative.
Seven nucleotide changes, probably representing polymorphisms, were identified in NS patients, unaffected family members and control persons (Table 4). Six of these sequence variants (SNPs) were found in intronic regions; only the 1131A→C substitution resided in the coding sequence (exon10), but did not alter the amino acid sequence.
We also searched for mutations in five sporadic patients with a diagnosis of cardio-facio-cutaneous (CFC) syndrome, which shares several phenotypic similarities with NS, as discussed below. No mutations in the PTPN11 gene were detected.
Discussion
Spectrum of PTPN11 mutations
In the present study we have analysed the entire coding sequence of the PTPN11 gene for mutations in 96 patients with NS, including 79 index cases and 17 relatives, and in five sporadic patients with CFC syndrome. A mutation was found in 23 (29%) of the 79 index patients which is less than the 45% reported by Tartaglia et al4, who however, argued that their mutation prevalence might be an overestimation because of a bias towards familial and linked cases. Both the mutation prevalence of 45% in familial cases (five out of 11) and 26% in sporadic cases (18 out of 68) are lower than the 59% and 37% in their study.4 For the familial cases, the difference can easily be explained by the fact that none of our families had been tested for linkage to this region. The lower mutation frequency observed in sporadic cases may be related to differences in the stringency of the diagnostic criteria.
All changes identified are missense mutations which result in non-conservative amino acid substitutions. In all families the mutation co-segregated with NS. Transmission of the mutation was maternal in three families and paternal in two families. In 16 out of 23 (70%) mutations involved exon 3 which codes for the N-SH2 domain. The most common mutation formerly reported by Tartaglia et al4, an A→G transition at position 922 in exon 8, was present in four index patients of this study, thus representing only 17% of the total number of mutations identified. Crystallographic data suggest that the N-SH2 domain has a key role in maintaining the inactive state of the SHP-2 protein.6 In this state, the N-SH2 and the PTP domains share a broad interaction surface. Several hydrogen bonds between the N-SH2/PTP domains occupy the most critical sites in catalysis. Significantly, most of the mutations found in our set of patients altered amino acids which are directly involved (Asn 58, Asp 61, Glu 69, Ala 72, Glu 76, Glu 79) in this complex network of bonds or around the interacting surface (Tyr 62, Tyr 63, Gln 256, Ile 282, Asn 308). The structure is further stabilised by polar interactions which involved other amino acids mutated in NS patients. An exchange of these critical amino acids might lead to the disturbance of the equilibrium between active and inactive forms of the SHP-2 protein.
The transition G→C in position 417 (Glu139Asp) is the only mutation identified which alters an amino acid in the C-SH2 domain. Although the C-SH2 domain alone is unlikely to contribute to activation,6 there is evidence that the C-SH2 domain contributes to the substrate specificity and to the binding affinity.7 Binding of the C-SH2 domain to a phosphotyrosine-containing ligand could increase the local ligand concentration so that the N-SH2 domain can bind a second site and adopts the active conformation. In this respect it is interesting to note that Glu139 is close to Arg138 and Ser140, which form hydrogen bonds with tyrosine phosphate of the ligands. How the mutation could affect the activity of the protein is yet unclear. It might result in a disequilibrium of substrate bound and unbound SHP-2 proteins.
Computational analyses based on the crystal structure predict that PTPN11 mutations in NS result in a gain of function. It is also possible that some of the mutations cause a dominant negative effect. Detailed molecular characterisation of the different mutations is needed to prove these assumptions.
Genotype–phenotype correlation
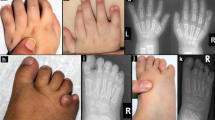
Diagnosis of NS is mainly based on craniofacial dysmorphism in addition to short stature, congenital heart defects, or mild mental retardation, but it is often hampered by the marked phenotypic variability of this syndrome. To date, the only comprehensive clinical data of NS patients with PTPN11 mutations were reported by Tartaglia et al4 but their study did not include craniofacial features. When analysing our 96 patients, we were surprised that several patients with facial abnormalities thought to be pathognomonic for NS (Figure 2) did not have a mutation in the PTPN11 gene. Moreover, widely varying phenotypes suggested that the group of patients without PTPN11 mutation was still genetically heterogeneous.
The most frequent craniofacial features in our patients with a mutation are low set ears in about 80%, followed by a low posterior hairline and down-slanting palpebral fissures both in 68% (Table 2). Hypertelorism, often regarded as one of the most important features of NS, was present in four out of five familiar index patients (80%) but in only one out of eight family members (12.5%) and in 54% of all patients with a mutation (Table 2). Since six out of eight affected family members are adults our findings confirm previous data showing that there is a tendency of some dysmorphic features, including hypertelorism, to normalise with increasing age.8,9 On average, each of the craniofacial features was present in 60% of patients, i.e. less often than in previous studies.10 However, not all craniofacial abnormalities in a single patient result in a characteristic NS face.
In patients with PNPN11 mutations, cryptorchidism was the most frequent clinical symptom, which was observed in 94% of the patients (Table 3). Ninety-three per cent of the patients with a mutation were short-statured. In NS patients the mean height parallels the 3rd centile in childhood, later on a delay of the growth spurt is frequently observed11. Because of the different ages at measurement of growth parameters in our patients it is difficult to compare these with other patient groups.
Cardiac defects were observed in 28 (87.5%) of 32 patients with a mutation. A comparison of our patients with PTPN11 mutations and those described by Tartaglia et al4 revealed that both groups have almost the same proportion of pulmonary stenosis and/or septal defects (Table 3). None of our patients carrying a PTPN11 mutation has a hypertrophic cardiomyopathy.
Mental retardation in patients with NS is usually mild and thought to occur in up to 35% of patients.10 We have observed mild mental retardation in 48% of patients with a mutation.
Pectus deformities, also regarded as a specific feature supporting the diagnosis of NS, were seen in 55% of patients, versus 78% reported by Tartaglia et al4 which might partly be due to the different definition, which in our case includes only pectus carinatum or excavatum, whereas a shield thorax is considered separately (Table 3).
Abnormalities of bleeding due to coagulation defects have frequently been described in NS patients. Out of the 72 individuals examined by Sharland et al,12 65% had abnormal bruising or bleeding, 40% prolonged activated partial thromboplastin-time, and in 50% of the patients abnormalities of the intrinsic pathway were found. Only one of our patients with a PTPN11 mutation was diagnosed as having mild von Willebrand syndrome, with complications of bleeding after surgery of cryptorchidism, but not after tonsillectomy. Two further patients with mutations had a moderate elongation of the activated partial thromboplastin-time and one patient reported easy bruising and longer bleeding after cutting, although coagulation studies gave normal results.
No obvious clinical differences were detected between subgroups of patients with mutations in different PTPN11 domains, although the number of patients was too small for a statistical analysis. There was only one sporadic patient with a G417C mutation in the C-SH2 domain (Figure 1) who presented with short stature and pulmonary stenosis. In addition, he had hepatosplenomegaly and leukocytosis of unknown origin since neonatal period. Abnormal proliferation of myelomonocytic cells may be as frequent as 10% in NS, and in the reported cases of NS-CMML the course of the disease seems to be relatively benign.13 In a retrospective study, Bader-Meunier et al14 reported four out of 51 patients with childhood myelodysplasia to have NS. The myelodysplasia resolved spontaneously in two of the four reported cases. Hepatosplenomegaly not related to cardiac failure is also a frequent finding in NS,12,15 and myelodysplastic disorders are often associated with a hepatosplenomegaly. The G417C mutation and a G417T mutation, both leading to a substitution of glutamine by asparagine in the C-SH2 domain were also present in two patients described by Tartaglia et al,4 but no further clinical information was given. None of the other patients with a mutation had myelodysplasia.
Cardio-facio-cutaneous (CFC) syndrome
CFC syndrome is a rare disorder with NS-like facial features, usually severe mental retardation, hyperkeratotic skin lesions, sparse, curly hair and absent eyebrows, heart defects, and sporadic occurrence.16 Because of the overlapping clinical features there is an ongoing discussion whether CFC syndrome and NS are allelic, or whether CFC is a contiguous gene syndrome.17,18 Although two patients with CFC were reported to have an interstitial deletion of 12q21-22,19,20 no deletions could be found in a further seven CFC patients investigated by Zollino et al,21 using the same probe employed by Rauen et al.19 Familial occurrence has been reported in two families.22,23 In a family with both CFC and NS, Schollen et al24 most recently identified a mutation in the PTPN11 gene. We screened five sporadic patients with CFC syndrome and did not find a mutation in the PTPN11 gene. This supports the notion that the CFC syndrome is genetically heterogeneous and that most cases represent a separate genetic disorder.
References
Jamieson, CR, van der Burgt, I & Brady, AF et al : Mapping a gene for Noonan syndrome to the long arm of chromosome 12. Nat Genet, (1994). 8, 357–360.
Legius, E, Schollen, E, Matthijs, G & Fryns, JP : Fine mapping of Noonan/cardio-facio-cutaneous syndrome in a large family. Eur J Hum Genet, (1998). 6, 32–37.
Tartaglia, M, Mehler, EL & Goldberg, R et al : Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nature, (2001). 29, 465–468.
Tartaglia, M, Kalidas, K & Shaw, A et al : PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet, (2002). 70, 1555–1563.
van der Burgt, I, Berends, E, Lommen, E, van Beersum, S, Hamel, B & Mariman, E : Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet, (1994). 53, 187–191.
Hof, P, Pluskey, S, Dhe-Paganon, S, Eck, MJ & Shoelson, SE : Crystal structure of the tyrosine phosphatase SHP-2. Cell, (1998). 92, 441–450.
Pluskey, S, Wandless, TJ, Walsh, CT & Shoelson, SE : Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem, (1995). 270, 2897–2900.
Allanson, JE, Hall, JG, Hughes, HE, Preus, M & Witt, RD : Noonan syndrome: the changing phenotype. Am J Med Genet, (1985). 21, 507–514.
Sharland, M, Burch, M, McKenna, WM & Patton, MA : A clinical study of Noonan syndrome. Arch Dis Child, (1992). 67, 178–183.
Gorlin, RJ, Cohen, MM & Hennekam, RCM : Syndromes of the Head and Neck. Oxford University Press (2001).
Ranke, MB, Heidemann, P, Knupfer, C, Enders, H, Schmaltz, AA & Bierich, JR : Noonan syndrome: growth and clinical manifestations in 144 cases. Eur J Pediatr, (1988). 148, 220–227.
Sharland, M, Patton, MA, Talbot, S, Chitolie, A & Bevan, DH : Coagulation-factor deficiencies and abnormal bleeding in Noonan's syndrome. Lancet, (1992). 339, 19–21.
Choong, K, Freedman, MH, Chitayat, D, Kelly, EN, Taylor, G & Zipursky, A : Juvenile myelomonocytic leukemia and Noonan syndrome. J Pediatr Hematol Oncol, (1999). 21, 523–527.
Bader-Meunier, B, Tchernia, G & Mielot, F et al : Occurrence of myeloproliferative disorder in patients with Noonan syndrome. J Pediatr, (1997). 130, 885–889.
George, CD, Patton, MA, el Sawi, M, Sharland, M & Adam, EJ : Abdominal ultrasound in Noonan syndrome: a study of 44 patients. Pediatr Radiol, (1993). 23, 316–318.
Reynolds, JF, Neri, G & Herrmann, JP et al : New multiple congenital anomalies/mental retardation syndrome with cardio-facio-cutaneous involvement – the CFC syndrome. Am J Med Genet, (1986). 25, 413–427.
Neri, G, Zollino, M & Rejnolds, JF : The Noonan-CFC controversy. Am J Med Genet, (1991). 39, 367–370.
Neri, G & Zollino, M : More on the Noonan-CFC controversy. Am J Med Genet, (1996). 65, 97–99.
Rauen, KA, Cotter, PD, Bitts, SM, Cox, VA & Golabi, M : Cardio-facio-cutaneous syndrome phenotype in an individual with an interstitial deletion of 12q: identification of a candidate region for CFC syndrome. Am J Med Genet, (2000). 93, 219–222.
Rauen, KA, Albertson, DG, Pinkel, D & Cotter, PD : Additional patient with del(12)(q21.2q22): Further evidence for a candidate region for cardio-facio-cutaneous syndrome?. Am J Med Genet, (2002). 110, 51–56.
Zollino, M & Neri, G : Partial deletion of chromosome 12q is not usually associated with CFC syndrome. Am J Med Genet, (2000). 95, 296
Fryns, JP, Volcke, P & van der Berghe, H : The cardio-facio-cutaneous (CFC) syndrome: autosomal dominant inheritance in a large family. Genet Couns, (1992). 3, 19–24.
Leichtman, LG : Are cardio-facio-cutaneous syndrome and Noonan syndrome distinct? A case of CFC offspring of a mother with Noonan syndrome. Clin Dysmorphol, (1996). 5, 61–64.
Schollen, E, Matthijs, G, Legius, E & Fryns, J : Mutation in the gene for protein tyrosine phosphatase SHP-2 (PTPN11) in a large family with Noonan/cardio-facio-cutaneous syndrome. Eur J Hum Genet, (2002). 10, Suppl. 1 238
Acknowledgements
The authors thank the patients and their families, and the patient group Noonan-Kinder eV Deutschland for their interest in our study. We also thank the clinicians and physicians for sending blood samples. B Royer-Pokora, T Goecke, S Wudy and B Göldner are thanked for their support. Thanks to K Hoffmann, U Fischer, H Madle and S Freier for technical assistance. This grant was supported by DHGP grant no. 01KW99087 to H-H Ropers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musante, L., Kehl, H., Majewski, F. et al. Spectrum of mutations in PTPN11 and genotype–phenotype correlation in 96 patients with Noonan syndrome and five patients with cardio-facio-cutaneous syndrome. Eur J Hum Genet 11, 201–206 (2003). https://doi.org/10.1038/sj.ejhg.5200935
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5200935
Keywords
This article is cited by
-
High frequency of hotspot mutation in PTPN11 gene among Moroccan patients with Noonan syndrome
Journal of Applied Genetics (2024)
-
Mutations in PTPN11 could lead to a congenital myasthenic syndrome phenotype: a Noonan syndrome case series
Journal of Neurology (2023)
-
A PTPN11 mutation in a woman with Noonan syndrome and protein-losing enteropathy
BMC Gastroenterology (2020)
-
Molecular and clinical studies in 107 Noonan syndrome affected individuals with PTPN11 mutations
BMC Medical Genetics (2020)
-
Molecular and environmental characterization of Noonan syndrome in Morocco reveals a significant association with consanguinity and advanced parental age
Egyptian Journal of Medical Human Genetics (2020)