Abstract
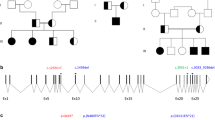
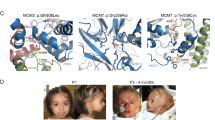
Börjeson–Forssman–Lehmann syndrome (BFLS; OMIM 301900) is characterized by moderate to severe mental retardation, epilepsy, hypogonadism, hypometabolism, obesity with marked gynecomastia, swelling of subcutaneous tissue of the face, narrow palpebral fissure and large but not deformed ears1. Previously, the gene associated with BFLS was localized to 17 Mb in Xq26–q27 (refs 2–4). We have reduced this interval to roughly 9 Mb containing more than 62 genes. Among these, a novel, widely expressed zinc-finger (plant homeodomain (PHD)-like finger) gene (PHF6) had eight different missense and truncation mutations in seven familial and two sporadic cases of BFLS. Transient transfection studies with PHF6 tagged with green fluorescent protein (GFP) showed diffuse nuclear staining with prominent nucleolar accumulation. Such localization, and the presence of two PHD-like zinc fingers, is suggestive of a role for PHF6 in transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others

References
Börjeson, M., Forssman, H. & Lehmann, O. An X-linked, recessively inherited syndrome characterized by grave mental deficiency, epilepsy, and endocrine disorder. Acta Med. Scand. 171, 13–21 (1962).
Gedeon, A.K. et al. Refinement of the background genetic map of Xq26-q27 and gene localization for Borjeson–Forssman–Lehmann Syndrome. Am. J. Med. Genet. 64, 63–68 (1996).
Mathews, K.D. et al. Linkage localization of Borjeson–Forssman–Lehmann syndrome. Am. J. Med. Genet. 34, 470–474 (1989).
Turner, G. et al. Borjeson–Forssman–Lehmann syndrome: clinical manifestations and gene localization to Xq26-27. Am. J. Med. Genet. 34, 463–469 (1989).
Skare, J.C. et al. Characterization of three overlapping deletions causing X-linked lymphoproliferative disease. Genomics 16, 254–255 (1993).
Chelly, J. & Mandel, J.-L. Monogenic causes of X-linked mental retardation. Nature Rev. Genet. 2, 669–680 (2001).
Gibbons, R.J., Picketts, D.J., Villard, L. & Higgs, D.R. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with α-thalassemia (ATR-X syndrome). Cell 80, 837–845 (1995).
Trivier, E. et al. Mutations in the kinase Rsk-2 associated with Coffin–Lowry syndrome. Nature 384, 567–570 (1996).
Amir, R.E. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet. 23, 185–188 (1999).
Stromme, P. et al. Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nature Genet. 30, 441–445 (2002).
Gustavson, K.H. et al. New X-linked syndrome with severe mental retardation, severely impaired vision, severe hearing defect, epileptic seizures, spasticity, restricted joint mobility, and early death. Am. J. Med. Genet. 45, 654–658 (1993).
Gedeon, A.K., Glass, I.A., Connor, J.M. & Mulley, J.C. Genetic localization of MRX27 to Xq24-26 defines another discrete gene for non-specific X-linked mental retardation. Am. J. Med. Genet. 64, 121–124 (1996).
Cabezas, D.A. et al. A new X linked mental retardation (XLMR) syndrome with short stature, small testes, muscle wasting, and tremor localises to Xq24-q25. J. Med. Genet. 37, 663–668 (2000).
Stevenson, R.E. Splitting and lumping in the nosology of XLMR. Am. J. Med. Genet. 97, 174–182 (2000).
Aasland, R., Gibson, T.J. & Stewart, A.F. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20, 56–59 (1995).
Meetei, A.R., Ullas, K.S. & Rao, M.R. Identification of two novel zinc-finger modules and nuclear localization signal in rat spermatidal protein TP2 by site-directed mutagenesis. J. Biol. Chem. 275, 38500–38507 (2000).
Dundr, M. & Misteli, T. Functional architecture in the cell nucleus. Biochem. J. 356, 297–310 (2001).
Pederson, T. & Politz, J.C. The nucleolus and the four ribonucleoproteins of translation. J. Cell. Biol. 148, 1091–1095 (2000).
Prives, C. & Hall, P.A. The p53 pathway. J. Pathol. 187, 112–126 (1999).
Hope, T.J. The ins and outs of HIV. Rev. Arch. Biochem. Biophys. 365, 186–191 (1999).
Zolotukhin, A.S. & Felber, B.K. Nucleoporins nup98 and nup214 participate in nuclear export of human immunodeficiency virus type 1. Rev. J. Virol. 73, 120–127 (1999).
Saha, V., Chaplin, T., Gregorini, A., Ayton, P. & Young, B.D. The leukemia-associated-protein (LAP) domain, a cysteine-rich motif, is present in a wide range of proteins, including MLL, AF10, and MLLT6 proteins. Proc. Natl Acad. Sci. USA 92, 9737–9741 (1995).
Bochar, D.A. et al. A family of chromatin remodeling factors related to Williams syndrome transcription factor. Proc. Natl Acad. Sci. USA 97, 1038–1043 (2000).
Loewith, R., Meijer, M., Lees-Miller, S.P., Riabowol, K. & Young, D. Three yeast proteins related to the human candidate tumor suppressor p33(ING1) are associated with histone acetyltransferase activities. Mol. Cell. Biol. 20, 3807–3816 (2000).
Dunwoodie, S.L., Henrique, D., Harrison, S.M. & Beddington, R.S.P. Mouse D113: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development 124, 3065–3076 (1997).
Gécz, J. et al. Gene structure and expression study of the SEDL gene for spondyloephiphyseal dysplasia tarda. Genomics 69, 242–251 (2000).
Acknowledgements
We thank the members of the families studied for their participation; C. Schwartz and J.P. Fryns for contributing samples; M. Lagerström Fermer for providing samples from the family affected with Gustavson syndrome; S. McDonnell and C. Derwas for help with cell culture; and M. Partington and G. Sutherland for critical reading of the manuscript. K.M.L. was supported by the Adelaide Research Scholarship from Adelaide University. This work was supported by the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Lower, K., Turner, G., Kerr, B. et al. Mutations in PHF6 are associated with Börjeson–Forssman –Lehmann syndrome. Nat Genet 32, 661–665 (2002). https://doi.org/10.1038/ng1040
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1040
This article is cited by
-
PHF6 maintains acute myeloid leukemia via regulating NF-κB signaling pathway
Leukemia (2023)
-
The Role of PHF6 in Hematopoiesis and Hematologic Malignancies
Stem Cell Reviews and Reports (2023)
-
Börjeson–Forssman–Lehmann syndrome: delineating the clinical and allelic spectrum in 14 new families
European Journal of Human Genetics (2023)
-
LncRNA Bmp1 promotes the healing of intestinal mucosal lesions via the miR-128-3p/PHF6/PI3K/AKT pathway
Cell Death & Disease (2021)
-
Loss of PHF6 leads to aberrant development of human neuron-like cells
Scientific Reports (2020)