Abstract
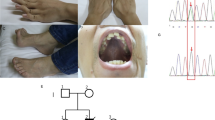
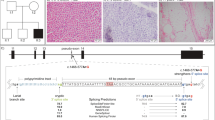
We have identified an unusual mitochondrial (mt) tRNA mutation in a seven year–old girl with a pure myopathy. This G to A transition at mtDNA position 15990 changed the anticodon normally found in proline tRNAs (UGG) to the one found in serine tRNAs (UGA), and is the first pathogenic anticodon alteration described in a higher eukaryote. The mutant mtDNA was heteroplasmic (85% mutant) in muscle but was undetectable in white blood cells from the patient and her mother. Analysis of single muscle fibres indicated that mutant mtDNAs severely impaired mitochondrial protein synthesis and respiratory chain activity, but only when present at greater than 90%. The recessive behaviour of this mtDNA alteration may explain the patient's relatively mild clinical phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wallace, D.C. Diseases of the mitochondrial DNA. Annu. Rev. Biochem. 61, 1175–1212 (1992).
Mita, S., Schmidt, B., Schon, E.A., DiMauro, S. & Bonilla, E. Detection of “deleted” mitochondrial genomes in cytochrome c oxidase-deficient muscle fibres of a patient with Keams-Sayre syndrome. Proc. natn. Acad. Sci. U.S.A. 86, 9509–9513 (1989).
Nakase, H. et al. Transcription and translation of deleted mitochondrial genomes in Kearns-Sayre syndrome: implications for pathogenesis. Am. J. hum. Genet. 46, 418–427 (1990).
Hayashi, J.-I. et al. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc. natn. Acad. Sci. U.S.A. 88, 10614–10618 (1991).
Moraes, C.T. et al. Molecular analysis of the muscle pathology associated with mitochondrial DNA deletions. Nature Genet. 1, 359–367 (1992).
Goto, Y.-I., Nonaka, I. & Horai, S. A mutation in the tRNALeu(UUR) gene associated with the ME LAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
Kobayashi, Y. et al. A point mutation in the mitochondrial tRNALeu(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem. Biophys. Res. Commun. 173, 816–822 (1990).
King, M.P., Koga, Y., Davidson, M. & Schon, E.A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNALeu(UUR) mutation associated with MELAS. Molec. cell Biol. 12, 480–490 (1992).
Chomyn, A. et al. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no changes in levels of upstream and downstream mature transcripts. Proc. natn. Acad. Sci. U.S.A. 89, 4221–4225 (1992).
Shoffner, J.M. et al. Myoclonic epilepsy and ragged-red fibre disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61, 931–937 (1990).
Yoneda, M. et al. A common mitochondrial DNA mutation in the t-RNALys of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem. Int. 21, 789–796 (1990).
Silvestri, G., Moraes, C.T., Shanske, S., Oh, S.J. & DiMauro, S. A new mtDNA mutation in the tRNALys gene associated with myoclonic epilepsy and ragged-red fibers (MERRF). Am. J. hum. Genet. 51, 1213–1217 (1992).
Zeviani, M. et al. A MERRF/MELAS overlap syndrome associated with a new point mutation of mitochondrial DNA tRNALys gene. Eur. J. hum. Genet. 1, 80–67 (1993).
Seibel, P. et al. Genetic, biochemical and pathophysiological characterization of a familial mitochondrial encephalomyopathy (MERRF). J. neurol. Sci. 105, 217–224 (1991).
Chomyn, A. et al. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Molec. cell. Biol. 11, 2236–2244 (1991).
Boulet, L., Karpati, G. & Shoubridge, E.A. Distribution and threshold expression of the tRNALLys mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers. Am. J. hum. Genet. 51, 1187–1200 (1992).
Moraes, C.T., Ricci, E., Bonilla, E., DiMauro, S. & Schon, E.A. The mitochondrial tRNALeu(UUR) mutation in MELAS: genetic, biochemical, and morphological correlations in skeletal muscle. Am. J. hum. Genet. 50, 934–949 (1992).
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
Attardi, G. & Schatz, G. Biogenesis of mitochondria. Annu. Rev. cell Biol. 4, 289–333 (1988).
Clayton, D.A. Replication and transcription of vertebrate mitochondrial DNA. Annu. Rev. cell Biol. 7, 453–478 (1991).
Kruse, B., Narasimhan, N. & Attardi, G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell 58, 391–397 (1989).
Sprinzl, M., Hartmann, T., Weber, J., Blank, J. & Zeidler, R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 17, r1–r172 (1989).
DiMauro, S. et al. Cytochrome c oxidase deficiency in Leigh syndrome. Ann. Neurol. 22, 498–506 (1987).
Hammans, S.R., Sweeney, M.G., Brockington, M., Morgan-Hughes, J.A. & Harding, A.E. Mitochondrial encephalopathies: molecular genetic diagnosis from blood samples. Lancet 337, 1311–1313 (1991).
Shoubridge, E.A., Karpati, G. & Hastings, K.E.M. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell 62, 43–49 (1990).
Ojala, D., Montoya, J. & Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 290, 470–474 (1981).
Leningher, A.L. Biochemistry. (Worth, New York, 1982).
Devereaux, J., Haeberli, P. & Smithies, O. A comprehensive set of sequence analysis programs for the VAX. Nucl. Acids Res. 12, 387–395 (1984).
Normanly, J. & Abelson, J. tRNA identity. Annu. Rev. Biochem. 58, 1029–1049 (1989).
Schulman, L.H. Recognition of tRNAs by aminoacyl-tRNA synthetases. Prog. nucl. acid Res. 41, 23–87 (1991).
Schulman, L.H. & Pelka, H. Anticodon switching changes the identity of methionine and valine tRNAs. Science 242, 765–768 (1988).
Schulman, L.H. & Pelka, H. An anticodon change switches the identity of E. Coli tRNA(mMet) from methionine to valine. Nucleic Acids Res. 18, 285–289 (1990).
Kumazawa, Y., Himeno, H., Miura, K.I. & Watanabe, K. Unilateral aminoacylation specificity between bovine mitochondria and eubacteria. J. Biochem. 109, 421–427 (1991).
Li, H., Cui, X. & Arnheim, N. Analysis of DNA sequence variation in single cells. Methods: a companion to Methods Enzymol. 2, 49–59 (1991).
Ruano, G. et al. Heat-soaked PCR: an efficient method for DNA amplification with applications to forensic analysis. BioTechniques 13, 266–274 (1992).
Tritschler, H.-J. et al. Mitochondrial myopathy of childhood associated with depletion of mitochondrial DNA. Neurology 42, 209–217 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moraes, C., Ciacci, F., Bonilla, E. et al. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nat Genet 4, 284–288 (1993). https://doi.org/10.1038/ng0793-284
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0793-284