Abstract
Mouse cartilage matrix deficiency (cmd) is an autosomal recessive mutation characterized by cleft palate, short limbs, tail and snout. Heterozygous mice show normal size and phenotype, while homozygous mice die just after birth due to respiratory failure. Biochemical and immunohistochemical characterization of cmd cartilage reveals normal levels of type II collagen and link protein, but an absence of the large cartilage proteoglycan, aggrecan. Here, we have mapped the aggrecan gene to a region of mouse chromosome 7 near the cmd locus. DNA sequencing of the aggrecan gene identified a 7 bp deletion in exon 5 resulting in a severely truncated molecule. The finding of an aggrecan mutation in the cmd mouse confirms the critical role of aggrecan in cartilage formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hardingham, T.E. & Fosang, A.J. Proteoglycans: many forms and many functions. FASEB J. 6, 861–870 (1992).
Rittenhouse, E. et al. Cartilage matrix deficiency (cmd): A new autosomal recessive lethal mutation in the mouse. J. Embryol. exp. Morph. 43, 71–84 (1978).
Kimata, K., Barrach, H.-J., Brown, K.S. & Pennypacker, J.P. Absence of proteoglycan core protein in cartilage from the cmd/cmd (cartilage matrix deficiency) mouse. J. biol. Chem. 256, 6961–6968 (1981).
Kimata, K. et al. Presence of link protein in cartilage from cmd/cmd (cartilage matrix deficiency) mice. Arch. Biochem. Biophys. 226, 506–516 (1983).
Doege, K.J., Sasaki, M., Horigan, E., Hassell, J.R. & Yamada, Y. Complete primary structure of the rat cartilage proteogly can core protein deduced from cDNA clones. J. biol. Chem. 262, 17757–17767 (1987).
Doege, K.J., Sasaki, M., Kimura, T. & Yamada, Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. J. biol. Chem. 266, 894–902 (1991).
Kozak, C.A. et al. Molecular genetic markers spanning mouse chromosome 10. Genomics 8, 519–524 (1990).
Adamson, M.C., Silver, J., & Kozak, C.A. The mouse homolog of the gibbon ape leukemia virus receptor: genetic mapping and a possible receptor function in rodents. Virology 183, 778–781 (1991).
Stoll, J., Kozak, C.A. & Goldman, D. Characterization and chromosomal mapping of a cDNA encoding tryptophan hydroxylase from a mouse mastocytoma cell line. Genomics 7, 88–96 (1990).
Danciger, M., Farber, D.B. & Kozak, C.A. Genetic mapping of three GABAA receptor-subunit genes in the mouse. Genomics 16, 361–365 (1993).
Kochhar, D.M. in Prevention of physical and mental congenital defects (ed. Marois, M.) 131–144 (Alan Liss, New York, 1985).
Doege, K. et al. Molecular biology of cartilage proteoglycan (aggrecan) and link protein in Extracellular matrix genes (eds Sandall J. & Boyd, D. P.)137–155 (Academic Press, Inc., New York,1990).
Argraves, W.S., McKeown-Longo, P.J. & Goetinck, P.F. Absence of proteoglycan core protein in the cartilage mutant nanomelia. FEBS Lett. 131, 265–268 (1981).
O'Donnell, C.M., Kaczman-Daniel, K.K., Goetinck, P.P. & Vertel, B.M. Nanomelic chondrocytes synthesize a glycoprotein related to chondroitin sulfate proteoglycan core protein. J. biol. Chem. 263, 17749–17754 (1988).
Stirpe, N.S., Argraves, W.S., & Goetinck, P.F. Chondrocytes from the cartilage proteoglycan-deficient mutant, nanomelia, synthesize greatly reduced levels of the proteoglycan core protein transcript. Dev. Biol. 124, 77–81 (1987).
Li, H. et al. cDNA cloning of chick cartilage chondroitin sulfate (aggrecan) core protein and identification of a stop codon in the aggrecan gene associated with the chondrodystrophy, nanomelia. J. biol. Chem. 268, 23504–23511 (1993).
Vandenberg, P. Molecular basis of heritable connective tissue disease. Biochem. Med. Metab. Biol. 49, 1–12 (1993).
Francomano, C.A. et al. The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics 1, 293–296 (1987).
Ahmad, N.N. et al. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc. natn. Acad. Sci. U.S.A. 88, 6624–6627 (1991).
Ahmad, N.N. et al. A second mutation in the Type II procollagen gene (COL2A1) causing Stickler syndrome (arthro-ophthalmopathy) is also a premature termination codon. Am. J. hum. Genet. 52, 39–45 (1993).
Lee, B., Vissing, H., Ramirez, F., Rogers, D. & Rimoin, D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science 244, 978–980 (1989).
Parotie, A. et al. Predisposition to familial osteoporosis linked to type II collagen gene. Lancet 1, 924–927 (1989).
Finkelstein, J.E. et al. Analysis of the chondroitin sulfate proteoglycan core protein (CSPGCP) gene in achondroplasia and pseudoachondroplasia. Amer. J. hum. Gen., 48, 97–102 (1991).
McKusik, V.A. Mendelian inheritance in man. 1176–1978 (Johns Hopkins University Press, Baltimore, 1992).
Takeda, M., Iwata, H., Suzuki, S., Brown, K.S. & Kimata, K. Correction of abnormal matrix formed by cmd/cmd chondrocytes in culture byexogenously added cartilage proteoglycan. J. Cell. Biol. 103, 1606–1614 (1986).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular cloning: A laboratory manual (Cold Spring Harbor Laboratory Press, New York, 1989).
Green, E.L. Linkage recombination and mapping in Genetics and probability in animal breeding experiments (ed. Green, E. L.) 77–113 (MacMillan, New York, 1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Watanabe, H., Kimata, K., Line, S. et al. Mouse cartilage matrix deficiency (cmd) caused by a 7 bp deletion in the aggrecan gene. Nat Genet 7, 154–157 (1994). https://doi.org/10.1038/ng0694-154
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0694-154
This article is cited by
-
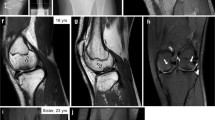
Novel missense ACAN gene variants linked to familial osteochondritis dissecans cluster in the C-terminal globular domain of aggrecan
Scientific Reports (2022)
-
Comparative Analysis of the Expression of Chondroitin Sulfate Subtypes and Their Inhibitory Effect on Axonal Growth in the Embryonic, Adult, and Injured Rat Brains
Tissue Engineering and Regenerative Medicine (2021)
-
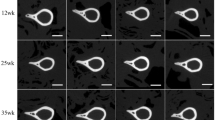
Evaluation of a new variant in the aggrecan gene potentially associated with chondrodysplastic dwarfism in Miniature horses
Scientific Reports (2020)
-
The effect of fibrillar degradation on the mechanics of articular cartilage: a computational model
Biomechanics and Modeling in Mechanobiology (2019)
-
Novel aggrecan variant, p. Gln2364Pro, causes severe familial nonsyndromic adult short stature and poor growth hormone response in Chinese children
BMC Medical Genetics (2018)