Abstract
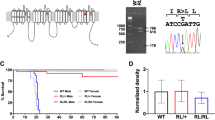
Hereditary hyperekplexia, an autosomal dominant neurologic disorder characterized by an exaggerated startle reflex and neonatal hypertonia, can be caused by mutations in the gene encoding the α1 subunit of the inhibitory glycine receptor (GLRA1). Spasmodic (spd), a recessive neurologic mouse mutant, resembles hyperekplexia phenotypically, and the two disease loci map to homologous chromosomal regions. Here we describe a Gira1 missense mutation in spd that results in reduced agonist sensitivity in glycine receptors expressed in vitro. We conclude that spd is a murine homologue of hyperekplexia and that mutations in GLRA1/Glra1 can produce syndromes with different inheritance patterns.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ryan, S.G. et al. Startle disease or hyperekplexia: response to clonazepam and assignment of the gene (STHE) to chromosome 5q by linkage analysis. Ann. Neurol. 31, 663–668 (1992).
Nigro, M.A. & Lim, H.-C.N. Hyperekplexia and sudden neonatal death. Pediatr. Neurol. 8, 221–225 (1992).
Giacoia, G.P. & Ryan, S.G. Am. J. Dis. Child, (in the press).
Andermann, F., Keene, D.L., Andermann, E. & Quesney, L.F. Startle disease or hyperekplexia: further delineation of the syndrome. Brain 103, 985–997 (1980).
Morley, D.J., Weaver, D.D. & Garg, B.P. Hyperexplexia: an inherited disorder of the startle response. Clin. Genet. 21, 388–396 (1982).
Ryan, S.G. et al. Genetic and radiation hybrid mapping of the hyperekplexia region on chromosome 5q. Am. J. hum. Genet. 51, 1334–1343 (1992).
Grenningloh, G. et al. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 238, 215–220 (1987).
Shiang, R. et al. Point mutations in the gene encoding the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nature Genet. 5, 351–358 (1993).
Chai, C.K. Hereditary spasticity in mice. J. Hered. 52, 241–243 (1961).
Lane, P.W., Ganser, A.L., Kerner, A.-L. & White, W.F., Spasmodic, a mutation on chromosome 11 in the mouse. J. Hered. 78, 353–356 (1987).
Becker, C.-M. Disorders of the inhibitory glycine receptor: the spastic mouse. FASEB J. 4, 2767–2774 (1990).
Buckwalter, M.S., Testa, C.M., Noebels, J.L. & Camper, S.A. Genetic mapping and evaluation of candidate genes for spasmodic, a neurological mouse mutation with abnormal startle response. Genomics 17, 279–286 (1993).
Heller, A.H. & Hallett, M. Eiectrophysiological studies with the spastic mutant mouse. Brain Res. 234, 299–308 (1982).
Cook, S.A. Research news. Mouse Genome 91, 117 (1993).
White, W.F. & Heller, A.H. Glycine receptor alteration in the mutant mouse spastic. Nature 298, 655–657 (1982).
Becker, C.-M., Hermans-Borgmeyer, I., Schmitt, B. & Betz, H. The glycine receptor deficiency of the mutant mouse spastic: evidence for normal glycine receptor structure and localization. J. Neurosci. 6, 1358–1364 (1986).
Eicher, E.M. & Lane, P. Assignment of LGXVI to chromosome 3 in the mouse. J. Hered. 71, 315–318 (1980).
Malosio, M.-L. et al. Alternative splicing generates two variants of the α1 subunit of the inhibitory glycine receptor. J. biol. Chem. 264, 2048–2053 (1991).
Grenningloh, G. et al. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 9, 771–776 (1990).
Vandenberg, R.J., Rajendra, S., French, C., Barry, P.H. & Schofield, P.R. The extracellular disulfide loop motif of the inhibitory glycine receptor does not form the agonist binding site. Molec. Pharmacol. 44, 198–203 (1993).
Langosch, D., Becker, C.-M. & Betz, H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur. J. Biochem, 194, 1–8 (1990).
Langosch, D., Thomas, L. & Betz, H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. natn. Acad. Sci. U.S.A. 85, 7394–7398 (1988).
Becker, C.-M., Schmieden, V., Tarroni, P., Strasser, U. & Betz, H. Isoform-selective deficit of glycine receptors in the mouse mutant spastic. Neuron 8, 283–289 (1992).
Schmieden, V., Grenningloh, G., Schofield, P.R. & Betz, H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 8, 695–700 (1989).
Sontheimer, H. et al. Functional chloride channels by mammalian expression of rat glycine receptor subunit. Neuron 2, 1491–1497 (1989).
Becker, C.-M., Hoch, W. & Betz, H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 7, 3717–3726 (1988).
Betz, H. & Becker, C.-M. The mammalian glycine receptor: biology and structure of a neuronal chloride channel protein. Neurochem. Int. 13, 137–146 (1988).
Langosch, D., Herbold, A., Schmieden, V., Borman, J. & Kirsch, J. Importance of Arg-219 for correct biogenesis of α1 homooligomeric glycine receptors. FEBS Lett. 336, 540–544 (1993).
Schofield, P.R., Pritchett, D.B., Sontheimer, H., Kettenmann, H. & Seeburg, P.H. Sequence and expression of human GABAA receptor α1 and β1 receptor subunits. FEBS Lett. 244, 361–364 (1989).
Shivers, B.D. et al. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron 3, 327–337 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ryan, S., Buckwalter, M., Lynch, J. et al. A missense mutation in the gene encoding the α1 subunit of the inhibitory glycine receptor in the spasmodic mouse. Nat Genet 7, 131–135 (1994). https://doi.org/10.1038/ng0694-131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0694-131
This article is cited by
-
LAMP5 in presynaptic inhibitory terminals in the hindbrain and spinal cord: a role in startle response and auditory processing
Molecular Brain (2019)
-
Glycine Receptor Deficiency and Its Effect on the Horizontal Vestibulo-ocular Reflex: a Study on the SPD1J Mouse
Journal of the Association for Research in Otolaryngology (2013)
-
Gating mechanisms in Cys-loop receptors
European Biophysics Journal (2009)
-
Nmf11 is a novel ENU-induced mutation in the mouse glycine receptor alpha 1 subunit
Mammalian Genome (2006)
-
Glycinergic transmission
Cell and Tissue Research (2006)