Abstract
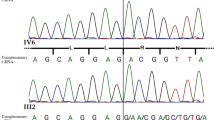
More than two hundred characterized 21–hydroxylase deficiency alleles appear to result exclusively from sequence exchanges involving the 21–hydroxylase gene (CYP21B) and a closely related pseudogene (CYP21A). Gene conversion–like events have also been reported in many other human gene clusters, but in the absence of a de novo mutation, the alternative explanation of a multiple recombination is possible. We now report a de novo pathological mutation at the 21–hydroxylase locus. DNA sequence analysis suggests that the mutation arose by a microconversion event involving exchange of up to 390 nucleotides between maternal CYP21A and CYP21B genes. This putative de novo gene conversion event appears to be the first characterized in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gitelman, S.E., Bristow, J. & Miller, W.L. Mechanism and consequences of the duplication of the human C4/P450c21/gene X locus. Molec. cell. Biol. 12, 2124–2134 (1992).
Strachan, T. Molecular genetics of congenital adrenal hyperplasia. Trends endocrin. Metab. 1, 68–72 (1989).
Strachan, T. & White, P.C. Molecular pathology of steroid 21-hydroxylase deficiency. J. Ster. Biochem. molec. Biol. 40, 537–543 (1991).
Collier, S. et al. Pulsed field gel electrophoresis identifies a high degree of variability in the number of tandem 21-hydroxylase and complement C4 repeats in 21-hydroxylase deficiency haplotypes. EMBO J. 8, 1393–1402 (1989).
Sinnott, P. et al. Genesis by meiotic unequal crossover of a de novo deletion that contributes to steroid 21-hydroxylase deficiency. Proc. natn. Acad. Sci. U.S.A. 87, 2107–2111 (1990).
Sinnott, P.J., Dyer, P.A., Price, D.A., Harris, R. & Strachan, T. 21-hydroxylase deficiency families with HLA-identical affected and unaffected sibs. J. med. Genet. 26, 10–17 (1989).
Collier, S., Tassabehji, M. & Strachan, T. A method for specific amplification and PCR sequencing of individual members of multigene families: application to the study of steroid 21-hydroxylase deficiency. PCR Meth. Applic. 1, 181–186 (1992).
Amor, M., Parker, K.L., Globerman, H., New, M.I. & White, P.C. Mutation in the CYP21B gene (lle-172→Asn) causes steroid 21 -hydroxylase deficiency. Proc. natn. Acad. Sci. U.S.A. 85, 1600–1604 (1988).
Chiou, S.-H., Hu, M.-C. & Chung, B.-C. A missense mutation at lle172→Asn or Arg356→ Trp causes steroid 21-hydroxylase deficiency. J. biol. Chem. 265, 3549–3552 (1990).
Tusie-Luna, M.-T., Traktman, P. & White, P.C. Determination of functional effects of mutations in the 21-hydroxylase gene (CYP21) using recombinant vaccinia virus. J. biol. Chem. 34, 20916–20922 (1990).
Kourilsky, P. Molecular mechanisms for gene conversion in higher cells. Trends Genet. 2, 60–63 (1986).
Wysocki, L.J. & Gefter, M.L. Gene conversion and the generation of antibody diversity. Ann. Rev. Biochem. 58, 509–531 (1989).
He, G.-S. & Grabowski, G.A. Gaucher's disease: a G+1 → A+1 IVS2 splice donor site mutation causing exon 2 skipping in the acid β-glucosidase mRNA. Am. J. hum. Genet. 51, 810–820 (1992).
Slightom, J.L., Blechi, A.E. & Smithies, O. Human fetal Gγ- and Aγ-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell 21, 627–638 (1980).
Bentley, D.L. & Rabbitts, T.H. Evolution of immunoglobulinV genes: evidence indicating that recently duplicated human Vkappa; sequences have diverged by gene conversion. Cell 32, 181–189 (1983).
Maeda, N. & Smithies, O. The evolution of multigene families: human haptoglobin genes. Ann. Rev. Genet. 20, 81–108 (1986).
Braun, L., Schneider, P.M., Giles, C.M., Bertrams, J., & Rottner, C. Null alleles of complement C4. Evidence for pseudogenes at the C4 locus and for gene conversion at the C4B locus. J. exp. Med. 171, 129–140 (1990).
Merritt, C.M., Easteal, S. & Board, P.G. Evolution of human α1-acid glycoprotein genes and surrounding Alu repeats. Genomics 6, 659–665 (1990).
Kuhner, M.K., Lawlor, D.A., Ennis, P.D. & Parham, P. Gene conversion in the evolution of the human and chimpanzee MHC class I loci. Tissue Antigens 38, 152–164 (1991).
Ehrlich, H.A. & Gyllenstein, U.B. Shared epitopes among HLA class II alleles: gene conversion, common ancestry and balancing selection. Immunol. Today 12, 412–414 (1991).
Taylor, J.B., Oliver, J., Sherrington, R. & Pemble, S.E. Structure of human glutathione S-transferase class Mu genes. Biochem J. 274, 587–593 (1991).
Huang, C.-H., Kikuchi, M., McCreary, J. & Blumenfeld, O.O. Gene conversion confined to a direct repeat of the acceptor splice site generates allelic diversity at human glycophorin (GYP locus). J. biol. Chem. 267, 3336–3342 (1992).
Deeb, S.S. et al. Genotpye-phenotype relationships in human red/green color-vision defects: molecular and psychophysical studies. Am. J. hum. Genet. 51, 687–700 (1992).
Hanioka, N., Kimura, S., Meyer, U.A. & Gonzalez, F.J. The human CYP2D locus associated with a common genetic defect in drug oxidation: a G1934 P A base change in intron 3 of a mutant CYP2D6 allele results in an aberrant 3′ splice recognition site. Am. J. hum. Genet. 47, 994–1001 (1990).
Higashi, Y. et al. Effects of indivdual mutations in the P-450 (C21) activity and their distribution in the patient genomes of congenital steroid 21-hydroxylase deficiency. J. Biochem. 109, 638–644 (1991).
Rodrigues, N.R. et al. Molecular characterization of the HLA-linked steroid 21 -hydroxylase B gene from an individual with congenital adrenal hyperplasia. EMBO J. 6, 1653–1661 (1987).
Harada, F., Kimura, A., Iwanaga, T., Shimozawa, K., Yata, J. & Sasazuki, T. Gene conversion-like events cause steroid 21-hydroxylase deficiency in congenital adrenal hyperplasia. Proc. natn. Acad. Sci. U.S.A. 84, 8091–8094 (1987).
Higashi, Y., Tanae, A., Inoue, H., Hiromasa, T., Fujii-Kuriyama, Y. Aberrant splicing and missense mutations cause steroid 21-hydroxylase [P450(c21)] deficiency in humans. Proc. natn. Acad. Sci. U.S.A. 85, 7486–7490 (1988).
Speiser, P.W., New, M.I. & White, P.C. Molecular genetic analysis of nonclassic steroid 21-hydroxylase deficiency associated with HLA-B14, DR1. New Engl J. Med. 319, 19–23 (1988).
Globerman, H., Amor, M., Parker, K.L., New, M.I. & White, P.C. Nonsense mutation causing steroid 21-hydroxylase deficiency. J. clin. Invest. 82, 139–144.
Tusie-Luna, M.-T., Speiser, P.W., Dumic, M., New, M.I. & White, P.C. A mutation (pro-30 to Leu) in CYP21 represents a potential nonclassic steroid 21-hydroxylase deficiency allele. Molec. Endochnol. 5, 685–692 (1991).
Mornet, E. et al. Distribution of deletions and seven point mutations on CYP21B genes in three clinical forms of 21-hydroxylase deficiency. Am. J. hum. Genet. 48, 79–88 (1991).
Owerbach, D., Ballard, A.-L. & Draznin, M.B. Salt-wasting congenital adrenal hyperplasia: detection and characterization of mutations in the steroid 21-hydroxylase gene, CYP21, using the polymerase chain reaction. J. clin. endoc. Metab. 74, 553–558 (1992).
Geliebter, J. & Nathenson, S.G. Recombination and the concerted evolution of the murine MHC. Trends Genet. 3, 107–112 (1987).
McCormack, W.T., Tjoelker, L.W. & Thompson, C.B. Avian B cell development: generation of an immunoglobulin repertoire by gene conversion. Ann. Rev. Immunol. 9, 219–241 (1991).
Loh, D.Y. & Baltimore, D. Sexual preference of apparent gene conversion events in MHC genes of mice. Nature 309, 639–640 (1984).
Krawinkel, U., Zoebelein, G. & Bothwell, A.L.M. Palindromic sequences are associated with sites of DNA breakage during gene conversion. Nucl. Acids Res. 14, 3871–3877 (1986).
Thein, S.L. & Wallace, R.B. The use of synthetic oligonucleotides as specific hybridization probes in the diagnosis of genetic disorders. In Human Genetic Disease: A Practical Approach, (ed. Davies, K.E.) 33–50 (IRL Press, Oxford, 1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Collier, S., Tassabehji, M. & Strachan, T. A de novo pathological point mutation at the 21–hydroxylase locus: implications for gene conversion in the human genome. Nat Genet 3, 260–265 (1993). https://doi.org/10.1038/ng0393-260
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0393-260
This article is cited by
-
Challenges of CYP21A2 genotyping in children with 21-hydroxylase deficiency: determination of genotype–phenotype correlation using next generation sequencing in Southeastern Anatolia
Journal of Endocrinological Investigation (2021)
-
A homozygous nonsense mutation in SOX9 in the dominant disorder campomelic dysplasia: a case of mitotic gene conversion
Human Genetics (2005)
-
Intense and highly localized gene conversion activity in human meiotic crossover hot spots
Nature Genetics (2004)
-
Ceruloplasmin gene defect associated with epilepsy in EL mice
Nature Genetics (1994)
-
Molecular pathology of 21‐hydroxylase deficiency
Journal of Inherited Metabolic Disease (1994)