Abstract
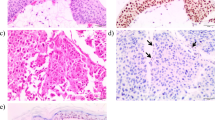
Because only a small fraction of asbestos-exposed individuals develop malignant mesothelioma1, and because mesothelioma clustering is observed in some families, we searched for genetic predisposing factors. We discovered germline mutations in the gene encoding BRCA1 associated protein-1 (BAP1) in two families with a high incidence of mesothelioma, and we observed somatic alterations affecting BAP1 in familial mesotheliomas, indicating biallelic inactivation. In addition to mesothelioma, some BAP1 mutation carriers developed uveal melanoma. We also found germline BAP1 mutations in 2 of 26 sporadic mesotheliomas; both individuals with mutant BAP1 were previously diagnosed with uveal melanoma. We also observed somatic truncating BAP1 mutations and aberrant BAP1 expression in sporadic mesotheliomas without germline mutations. These results identify a BAP1-related cancer syndrome that is characterized by mesothelioma and uveal melanoma. We hypothesize that other cancers may also be involved and that mesothelioma predominates upon asbestos exposure. These findings will help to identify individuals at high risk of mesothelioma who could be targeted for early intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Carbone, M. et al. Malignant mesothelioma: Facts, myths and hypotheses. J. Cell. Physiol., doi:10.1002/jcp.22724 (published online 16 March 2011).
Flores, R.M. et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J. Thorac. Cardiovasc. Surg. 135, 620–626 (2008).
Tweedale, G. Asbestos and its lethal legacy. Nat. Rev. Cancer 2, 311–315 (2002).
Kamp, D.W. Asbestos-induced lung diseases: an update. Transl. Res. 153, 143–152 (2009).
Moolgavkar, S.H., Meza, R. & Turim, J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973–2005. Cancer Causes Control 20, 935–944 (2009).
Goldberg, M. et al. The French National Mesothelioma Surveillance Program. Occup. Environ. Med. 63, 390–395 (2006).
Britton, M. The epidemiology of mesothelioma. Semin. Oncol. 29, 18–25 (2002).
Burki, T. Health experts concerned over India's asbestos industry. Lancet 375, 626–627 (2010).
Pan, X.L., Day, H.W., Wang, W., Beckett, L.A. & Schenker, M.B. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am. J. Respir. Crit. Care Med. 172, 1019–1025 (2005).
Carbone, M. et al. Erionite exposure in North Dakota and in the Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA published online, doi:10.1073/pnas.1105887108 (25 July 2011).
Carbone, M. et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat. Rev. Cancer 7, 147–154 (2007).
Flejter, W.L., Li, F.P., Antman, K.H. & Testa, J.R. Recurring loss involving chromosomes 1, 3, and 22 in malignant mesothelioma: possible sites of tumor suppressor genes. Genes Chromosom. Cancer 1, 148–154 (1989).
Lu, Y.Y., Jhanwar, S.C., Cheng, J.Q. & Testa, J.R. Deletion mapping of the short arm of chromosome 3 in human malignant mesothelioma. Genes Chromosom. Cancer 9, 76–80 (1994).
Murthy, S.S. & Testa, J.R. Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J. Cell. Physiol. 180, 150–157 (1999).
Jensen, D.E. et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 16, 1097–1112 (1998).
Ventii, K.H. et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 68, 6953–6962 (2008).
Das, S. et al. Diverse mutations in patients with Menkes disease often lead to exon skipping. Am. J. Hum. Genet. 55, 883–889 (1994).
Baralle, D. & Baralle, M. Splicing in action: assessing disease causing sequence changes. J. Med. Genet. 42, 737–748 (2005).
Harbour, J.W. et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330, 1410–1413 (2010).
Houlston, R.S. & Damato, B.E. Genetic predisposition to ocular melanoma. Eye 13, 43–46 (1999).
Bott, M. et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 43, 668–672 (2011).
Albert, D.M. & Van Buren, J.J. Intraocular melanomas. in Cancer, Principles & Practice of Oncology, Vol. 1 (eds. De Vita, V.T., Lawrence, T.S. & Rosenberg, S.A.) 2090–2098 (Lippincott Williams & Wilkins, Philadelphia, Pennsylvania, USA, 2011).
Korn, J.M. et al. Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat. Genet. 40, 1253–1260 (2008).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
International HapMap Consortium. A second generation human haplotype map of over 3.1 million SNPs. Nature 449, 851–861 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Mäkinen, V.P. et al. High-throughput pedigree drawing. Eur. J. Hum. Genet. 13, 987–989 (2005).
Gudbjartsson, D.F., Thorvaldsson, T., Kong, A., Gunnarsson, G. & Ingolfsdottir, A. Allegro version 2. Nat. Genet. 37, 1015–1016 (2005).
Altomare, D.A. et al. Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc. Natl. Acad. Sci. USA 106, 3420–3425 (2009).
Yang, H. et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl. Acad. Sci. USA 107, 12611–12616 (2010).
Cheung, M. et al. The promyelocytic leukemia zinc finger gene, PLZF, is frequently downregulated in malignant mesothelioma cells and contributes to cell survival. Oncogene 29, 1633–1640 (2010).
Acknowledgements
We are grateful to the affected individuals and their families for their participation in this study. We thank M. Bianchi, C. Menges, I. Pagano, J.D. Rowley and D.C. Ward for advice and review of the manuscript. We also thank J. Talarchek and R. Harrington for technical assistance, K. Aquino-Michaels for assisting in the collection of samples and clinical information, S.C. Jhanwar for providing some of the mesothelioma cell lines, A. Vachani for blood and tissue samples from one member of the L family and B. Luo for providing exome sequence data. This work was supported by US National Institutes of Health (NIH) grants P01CA-114047, P30CA-06927 and P30CA-71789, by the AACR-Landon Award for International Collaboration in Cancer Research to M. Carbone, N.J.C., H.I.P., J.R.T., M.T. and H.Y., and by the Local No. 14 Mesothelioma Fund of the International Association of Heat and Frost Insulators & Allied Workers to J.R.T.
Author information
Authors and Affiliations
Contributions
J.R.T. led the team at Fox Chase Cancer Center (FCCC; M. Cheung, J.P., Y.T. and E.S.) that first identified and characterized the BAP1 mutations and genomic alterations in the two families with high incidence of mesothelioma, performed the splicing and functional assays, and discovered BAP1 mutations in sporadic tumors and cell lines. N.J.C. designed and directed the genetic linkage analysis studies performed by J.E.B. H.I.P. treated many of these patients and together with S.T. and M.H. provided the tumor samples, DNA and clinical information. A.U.D. performed the mineralogical studies. M. Carbone conceived the project, assembled the families and the entire research group, diagnosed mesotheliomas and led the team at University of Hawaii Cancer Center (UHCC; M.N., A.P., Z.R., S.C., M.T., G.G. and H.Y.) that confirmed the mutations in the two families and discovered germline and somatic mutations in sporadic mesotheliomas. M.N. led the experimental work conducted by the UHCC team. J.R.T. and M. Carbone wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2 and Supplementary Figures 1–6 (PDF 5179 kb)
Rights and permissions
About this article
Cite this article
Testa, J., Cheung, M., Pei, J. et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 43, 1022–1025 (2011). https://doi.org/10.1038/ng.912
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.912
This article is cited by
-
Mutational signature of extracranial meningioma metastases and their respective primary tumors
Acta Neuropathologica Communications (2023)
-
SETDB1 tumour suppressor roles in near-haploid mesothelioma involve TP53
British Journal of Cancer (2023)
-
Targeting BAP1 with small compound inhibitor for colon cancer treatment
Scientific Reports (2023)
-
BAP1 as a guardian of genome stability: implications in human cancer
Experimental & Molecular Medicine (2023)
-
Deficiency of BAP1 inhibits neuroblastoma tumorigenesis through destabilization of MYCN
Cell Death & Disease (2023)