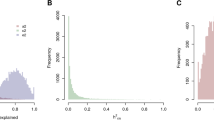
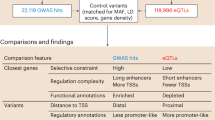
Abstract
Sequence-based variation in gene expression is a key driver of disease risk. Common variants regulating expression in cis have been mapped in many expression quantitative trait locus (eQTL) studies, typically in single tissues from unrelated individuals. Here, we present a comprehensive analysis of gene expression across multiple tissues conducted in a large set of mono- and dizygotic twins that allows systematic dissection of genetic (cis and trans) and non-genetic effects on gene expression. Using identity-by-descent estimates, we show that at least 40% of the total heritable cis effect on expression cannot be accounted for by common cis variants, a finding that reveals the contribution of low-frequency and rare regulatory variants with respect to both transcriptional regulation and complex trait susceptibility. We show that a substantial proportion of gene expression heritability is trans to the structural gene, and we identify several replicating trans variants that act predominantly in a tissue-restricted manner and may regulate the transcription of many genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Eichler, E.E. et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 11, 446–450 (2010).
Cheung, V.G. et al. Mapping determinants of human gene expression by regional and genome-wide association. Nature 437, 1365–1369 (2005).
Göring, H.H. et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nat. Genet. 39, 1208–1216 (2007).
Grundberg, E. et al. Population genomics in a disease targeted primary cell model. Genome Res. 19, 1942–1952 (2009).
Stranger, B.E. et al. Population genomics of human gene expression. Nat. Genet. 39, 1217–1224 (2007).
Myers, A.J. et al. A survey of genetic human cortical gene expression. Nat. Genet. 39, 1494–1499 (2007).
Schadt, E.E. et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 6, e107 (2008).
Emilsson, V. et al. Genetics of gene expression and its effect on disease. Nature 452, 423–428 (2008).
Dimas, A.S. et al. Common regulatory variation impacts gene expression in a cell type–dependent manner. Science 325, 1246–1250 (2009).
Greenawalt, D.M. et al. A survey of the genetics of stomach, liver, and adipose gene expression from a morbidly obese cohort. Genome Res. 21, 1008–1016 (2011).
Nica, A.C. et al. The architecture of gene regulatory variation across multiple human tissues: the MuTHER study. PLoS Genet. 7, e1002003 (2011).
Zeller, T. et al. Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS ONE 5, e10693 (2010).
Ding, J. et al. Gene expression in skin and lymphoblastoid cells: refined statistical method reveals extensive overlap in cis-eQTL signals. Am. J. Hum. Genet. 87, 779–789 (2010).
Nica, A.C. et al. Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS Genet. 6, e1000895 (2010).
Dixon, A.L. et al. A genome-wide association study of global gene expression. Nat. Genet. 39, 1202–1207 (2007).
Price, A.L. et al. Single-tissue and cross-tissue heritability of gene expression via identity-by-descent in related or unrelated individuals. PLoS Genet. 7, e1001317 (2011).
Spector, T.D. & Williams, F.M. The UK Adult Twin Registry (TwinsUK). Twin Res. Hum. Genet. 9, 899–906 (2006).
Andrew, T. et al. Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res. 4, 464–477 (2001).
Visscher, P.M., Benyamin, B. & White, I. The use of linear mixed models to estimate variance components from data on twin pairs by maximum likelihood. Twin Res. 7, 670–674 (2004).
Storey, J.D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100, 9440–9445 (2003).
Aulchenko, Y.S. et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 41, 47–55 (2009).
Freathy, R.M. et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat. Genet. 42, 430–435 (2010).
Brown, K.M. et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat. Genet. 40, 838–840 (2008).
Sulem, P. et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 39, 1443–1452 (2007).
Anderson, C.A., Soranzo, N., Zeggini, E. & Barrett, J.C. Synthetic associations are unlikely to account for many common disease genome-wide association signals. PLoS Biol. 9, e1000580 (2011).
Dickson, S.P. et al. Rare variants create synthetic genome-wide associations. PLoS Biol. 8, e1000294 (2010).
Powell, J.E. et al. Genetic control of gene expression in whole blood and lymphoblastoid cell lines is largely independent. Genome Res. 22, 456–466 (2012).
Heinig, M. et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 467, 460–464 (2010).
Small, K.S. et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat. Genet. 43, 561–564 (2011).
Humbert, N. et al. Regulation of ploidy and senescence by the AMPK-related kinase NUAK1. EMBO J. 29, 376–386 (2010).
Li, H., Ruan, J. & Durbin, R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 18, 1851–1858 (2008).
Teo, Y.Y. et al. A genotype calling algorithm for the Illumina BeadArray platform. Bioinformatics 23, 2741–2746 (2007).
Aulchenko, Y.S., de Koning, D.J. & Haley, C. Genomewide rapid association using mixed model and regression: a fast and simple method for genomewide pedigree-based quantitative trait loci association analysis. Genetics 177, 577–585 (2007).
Chen, W.M. & Abecasis, G.R. Family-based association tests for genomewide association scans. Am. J. Hum. Genet. 81, 913–926 (2007).
Aulchenko, Y.S., Ripke, S., Isaacs, A. & van Duijn, C.M. GenABEL: an R library for genome-wide association analysis. Bioinformatics 23, 1294–1296 (2007).
Aulchenko, Y.S., Struchalin, M.V. & van Duijn, C.M. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics 11, 134 (2010).
R Development Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, Vienna, 2010).
McVean, G.A. et al. The fine-scale structure of recombination rate variation in the human genome. Science 304, 581–584 (2004).
Bates, D.M. lme4: Linear Mixed-Effects Models Using S4 Classes. (R Foundation for Statistical Computing, Vienna, 2010).
Abecasis, G.R., Cherny, S.S., Cookson, W.O. & Cardon, L.R. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 30, 97–101 (2002).
Min, J.L. et al. The use of genome-wide eQTL associations in lymphoblastoid cell lines to identify novel genetic pathways involved in complex traits. PLoS ONE 6, e22070 (2011).
Golding, J., Pembrey, M. & Jones, R. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr. Perinat. Epidemiol. 15, 74–87 (2001).
Li, Y. et al. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 34, 816–834 (2010).
Acknowledgements
The MuTHER Study was funded by a program grant from the Wellcome Trust (081917/Z/07/Z) and by core funding for the Wellcome Trust Centre for Human Genetics (090532). Additional funding came from the European Community's Seventh Framework Programme (FP7/2007-2013), ENGAGE project and grant agreement HEALTH-F4-2007-201413, the Swiss National Science Foundation and the NCCR Frontiers in Genetics, the Louis-Jeantet Foundation and a US National Institutes of Health–NIMH grant (GTEx project). Additional details on the funding for the participating studies and investigators are provided in the Supplementary Note.
Author information
Authors and Affiliations
Consortia
Contributions
K.R.A., M.I.M., P.D., E.T.D. and T.D.S. conceived the study. E.G., K.S.S., Å.K.H., A.C.N., A. Buil and S.K. analyzed data. T.-P.Y., E.M., S.-Y.S., J.L.M., K.T.Z., S.R., K.H., G.T., A.K., U.T., S.P., N.S., E.E.S., K.S. and G.D.S. contributed reagents, materials, or analysis tools. A. Barrett, J.N., M.S., A.W., D.G., M.T., N.H., C.I., M.K. and G.S. performed wet lab experiments or collected samples. J.T.B., C.A., A.S.D., D.K., C.E.L., P.D.M., S.B.M., L.P., L.T., S.T., V.B., R.D., F.O.N., S.O. and C.M.L. contributed experimental and technical support as well as discussion. E.G. prepared the manuscript, with contributions from K.S.S., Å.K.H., A.C.N., A. Buil, M.I.M., P.D., E.T.D. and T.D.S. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members and affiliations is provided in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Supplementary Tables 1–10 and Supplementary Note (PDF 1816 kb)
Rights and permissions
About this article
Cite this article
Grundberg, E., Small, K., Hedman, Å. et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet 44, 1084–1089 (2012). https://doi.org/10.1038/ng.2394
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2394
This article is cited by
-
Transposable elements mediate genetic effects altering the expression of nearby genes in colorectal cancer
Nature Communications (2024)
-
Identification of asthma-related genes using asthmatic blood eQTLs of Korean patients
BMC Medical Genomics (2023)
-
Genetic variation in cis-regulatory domains suggests cell type-specific regulatory mechanisms in immunity
Communications Biology (2023)
-
The contributions of mitochondrial and nuclear mitochondrial genetic variation to neuroticism
Nature Communications (2023)
-
Maximizing the value of twin studies in health and behaviour
Nature Human Behaviour (2023)