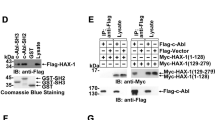
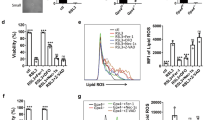
Abstract
The Fanconi anemia group C protein (FANCC) plays an important role in hematopoiesis by ensuring the survival of hematopoietic progenitor cells through an unknown mechanism. We investigated the function of FANCC by identifying FANCC-binding proteins in hematopoietic cells. Here we show that glutathione S-transferase P1-1 (GSTP1) interacts with FANCC, and that overexpression of both proteins in a myeloid progenitor cell line prevents apoptosis following factor deprivation. FANCC increases GSTP1 activity after the induction of apoptosis. GSTP1 is an enzyme that catalyzes the detoxification of xenobiotics and by-products of oxidative stress, and it is frequently upregulated in neoplastic cells. Although FANCC lacks homology with conventional disulfide reductases, it functions by preventing the formation of inactivating disulfide bonds within GSTP1 during apoptosis. The prevention of protein oxidation by FANCC reveals a novel mechanism of enzyme regulation during apoptosis and has implications for the treatment of degenerative diseases with thiol reducing agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garcia-Higuera, I., Kuang, Y. & D'Andrea, A.D. The molecular and cellular biology of Fanconi anemia. Curr. Opin. Hematol. 6, 83–88 (1999).
Joenje, H. & Gille, J.J.P. Oxygen metabolism and chromosomal breakage in Fanconi anemia. in Fanconi Anemia: Clinical, Cytogenetic, and Experimental Aspects (eds. Auerbach, A.D., Schroeder-Kurth, T.M. & Obe, G.) 174–182 (Springer, Berlin, 1989).
Joenje, H. et al. Complementation analysis in Fanconi anemia: assignment of the reference FA-H patient to group A. Am. J. Hum. Genet. 67, 759–762 (2000).
de Winter, J.P. et al. The Fanconi anemia protein FANCF forms a nuclear complex with FANCA, FANCC and FANCG. Hum. Mol. Genet. 9, 2665–2674 (2000).
Medhurst, A.L., Huber, P.A., Waisfisz, Q., de Winter, J.J. & Mathew, C.G. Direct interactions of the five known Fanconi anaemia proteins suggest a common functional pathway. Hum. Mol. Genet. 10, 423–429 (2001).
Garcia-Higuera, I. et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell. 7, 249–262 (2001).
Youssoufian, H. Localization of Fanconi anemia C protein to the cytoplasm of mammalian cells. Proc. Natl. Acad. Sci. USA 91, 7975–7979 (1994).
Youssoufian, H. Cytoplasmic localization of FAC is essential for the correction of a prerepair defect in Fanconi anemia group C cells. J. Clin. Invest. 97, 2003–2010 (1996).
Kruyt, F.A. et al. Abnormal microsomal detoxification implicated in Fanconi anemia group C by interaction of the FAC protein with NADPH cytochrome P450 reductase. Blood 92, 3050–3056 (1998).
Hoshino, T. et al. Molecular chaperone GRP94 binds to the Fanconi anemia group C protein and regulates its intracellular expression. Blood 91, 4379–4386 (1998).
Hoatlin, M.E. et al. A novel BTB/POZ transcriptional repressor protein interacts with the Fanconi anemia group C protein and PLZF. Blood 94, 3737–3747 (1999).
Cumming, R.C., Liu, J.M., Youssoufian, H. & Buchwald, M. Suppression of apoptosis in hematopoietic factor-dependent progenitor cell lines by expression of the FAC gene. Blood 88, 4558–4567 (1996).
Wang, J. et al. Overexpression of the fanconi anemia group C gene (FAC) protects hematopoietic progenitors from death induced by Fas-mediated apoptosis. Cancer Res. 58, 3538–3541 (1998).
Rathbun, R.K. et al. Inactivation of the Fanconi anemia group C gene augments interferon- gamma-induced apoptotic responses in hematopoietic cells. Blood 90, 974–985 (1997).
Haneline, L.S. et al. Multiple inhibitory cytokines induce deregulated progenitor growth and apoptosis in hematopoietic cells from Fac−/− mice. Blood 91, 4092–4098 (1998).
Otsuki, T. et al. Tumor necrosis factor-α and CD95 ligation suppress erythropoiesis in Fanconi anemia C gene knockout mice. J. Cell. Physiol. 179, 79–86 (1999).
Kodym, R., Calkins, P. & Story, M. The cloning and characterization of a new stress response protein. A mammalian member of a family of theta class glutathione S-transferase-like proteins. J. Biol. Chem. 274, 5131–5137 (1999).
Voehringer, D.W. et al. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc. Natl. Acad. Sci. USA 97, 2680–2685 (2000).
Kampranis, S.C. et al. A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. J. Biol. Chem. 275, 29207–29216 (2000).
Hayes, J.D. & Pulford, D.J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 30, 445–600 (1995).
Hayes, J.D. & McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 31, 273–300 (1999).
Meister, A. Selective modification of glutathione metabolism. Science 220, 472–477 (1983).
van den Dobbelsteen, D.J. et al. Rapid and specific efflux of reduced glutathione during apoptosis induced by anti-Fas/APO-1 antibody. J. Biol. Chem. 271, 15420–15427 (1996).
Bojes, H.K. et al. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem. J. 325, 315–319 (1997).
Bojes, H.K., Feng, X., Kehrer, J.P. & Cohen, G.M. Apoptosis in hematopoietic cells (FL5.12) caused by interleukin-3 withdrawal: relationship to caspase activity and the loss of glutathione. Cell. Death Differ. 6, 61–70 (1999).
Shen, H. et al. Modulation of class π glutathione transferase activity by sulfhydryl group modification. Arch. Biochem. Biophys. 286, 178–182 (1991).
Shen, H., Tsuchida, S., Tamai, K. & Sato, K. Identification of cysteine residues involved in disulfide formation in the inactivation of glutathione transferase P-form by hydrogen peroxide. Arch. Biochem. Biophys. 300, 137–141 (1993).
Terada, T. et al. Modulation of glutathione S-transferase activity by a thiol/disulfide exchange reaction and involvement of thioltransferase. Arch. Biochem. Biophys. 300, 495–500 (1993).
Berhane, K., Widersten, M., Engstrom, A., Kozarich, J.W. & Mannervik, B. Detoxication of base propenals and other α, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc. Natl. Acad. Sci. USA 91, 1480–1484 (1994).
Shea, T.C., Kelley, S.L. & Henner, W.D. Identification of an anionic form of glutathione transferase present in many human tumors and human tumor cell lines. Cancer Res. 48, 527–533 (1988).
Sato, K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv. Cancer Res. 52, 205–255 (1989).
Cuozzo, J.W. & Kaiser, C.A. Competition between glutathione and protein thiols for disulphide-bond formation. Nature Cell Biol. 1, 130–135 (1999).
Frand, A.R., Cuozzo, J.W. & Kaiser, C.A. Pathways for protein disulphide bond formation. Trends Cell Biol. 10, 203–210 (2000).
Wong, J.C.Y., Alon, N. & Buchwald, M. Cloning of the bovine and rat Fanconi anemia group C cDNA. Mamm. Genome 8, 522–525 (1997).
Lay, A.J. et al. Phosphoglycerate kinase acts in tumour angiogenesis as a disulphide reductase. Nature 408, 869–873 (2000).
Joenje, H., Arwert, F., Eriksson, A.W., de Koning, H. & Oostra, A.B. Oxygen-dependence of chromosomal aberrations in Fanconi's anaemia. Nature 290, 142–143 (1981).
Ruppitsch, W., Meisslitzer, C., Hirsch-Kauffmann, M. & Schweiger, M. Overexpression of thioredoxin in Fanconi anemia fibroblasts prevents the cytotoxic and DNA damaging effect of mitomycin C and diepoxybutane. FEBS Lett. 422, 99–102 (1998).
Herzenberg, L.A. et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 94, 1967–1972 (1997).
Adler, V., Yin, Z., Tew, K.D. & Ronai, Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18, 6104–6111 (1999).
Wilhelm, D., Bender, K., Knebel, A. & Angel, P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol. Cell Biol. 17, 4792–4800 (1997).
Adler, V. et al. Regulation of JNK signaling by GSTp. EMBO J. 18, 1321–1334 (1999).
Yin, Z., Ivanov, V.N., Habelhah, H., Tew, K. & Ronai, Z. Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 60, 4053–4057 (2000).
Rothe, M., Pan, M.G., Henzel, W.J., Ayres, T.M. & Goeddel, D.V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83, 1243–1252 (1995).
Habig, W.H., Pabst, M.J. & Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974).
Reed, D.J. et al. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem 106, 55–62 (1980).
Acknowledgements
We thank J. Liu for providing the FANCC retroviral vector, G. Boulianne for comments on the manuscript and D. Riddick for technical advice. This research was supported by the Canadian Institutes of Health Research, the National Institutes of Health (HL52138) and the Burroughs Wellcome Clinical Scientist Award (to H.Y.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cumming, R., Lightfoot, J., Beard, K. et al. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med 7, 814–820 (2001). https://doi.org/10.1038/89937
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/89937
This article is cited by
-
Hydrogen gas protects IP3Rs by reducing disulfide bridges in human keratinocytes under oxidative stress
Scientific Reports (2017)
-
Increased red cell distribution width in Fanconi anemia: a novel marker of stress erythropoiesis
Orphanet Journal of Rare Diseases (2016)
-
Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp
Cancer Cell International (2015)
-
Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death
Cancer Chemotherapy and Pharmacology (2015)
-
Improvement of genetic stability in lymphocytes from Fanconi anemia patients through the combined effect of α-lipoic acid and N-acetylcysteine
Orphanet Journal of Rare Diseases (2012)