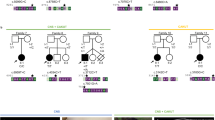
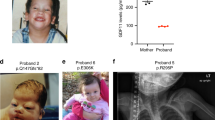
Abstract
All vertebrates display a characteristic asymmetry of internal organs with the cardiac apex, stomach and spleen towards the left, and the liver and gall bladder on the right1,2,3. Left-right (L-R) axis abnormalities or laterality defects are common in humans (1 in 8,500 live births). Several genes (such as Nodal, Ebaf and Pitx2) have been implicated in L-R organ positioning in model organisms2,3,4. In humans, relatively few genes have been associated with a small percentage of human situs defects. These include ZIC3 (ref. 5), LEFTB (formerly LEFTY2; ref. 6) and ACVR2B (encoding activin receptor IIB; ref. 7). The EGF-CFC genes8, mouse Cfc1 (encoding the Cryptic protein; ref. 9) and zebrafish one-eyed pinhead (oep; refs 10, 11) are essential for the establishment of the L-R axis12,13. EGF-CFC proteins act as co-factors for Nodal-related signals11, which have also been implicated in L-R axis development4. Here we identify loss-of-function mutations in human CFC1 (encoding the CRYPTIC protein) in patients with heterotaxic phenotypes (randomized organ positioning). The mutant proteins have aberrant cellular localization in transfected cells and are functionally defective in a zebrafish oep-mutant rescue assay. Our findings indicate that the essential role of EGF-CFC genes and Nodal signalling in left-right axis formation is conserved from fish to humans. Moreover, our results support a role for environmental and/or genetic modifiers in determining the ultimate phenotype in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kosaki, K. & Casey, B. Genetics of human left-right axis malformations. Cell Dev. Biol. 9, 89 –99 (1998).
Capdevila, J., Vogan, K.J., Tabin, C. & Izpisua Belmonte, J.C. Mechanisms of left-right determination in vertebrates. Cell 101 , 9–21 (2000).
Burdine, R. & Schier, A.F. Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 14, 763–776 (2000).
Schier, A.F. & Shen, M.M. Nodal signalling in vertebrate development. Nature 403, 385– 389 (2000).
Gebbia, M. et al. X-linked situs abnormalities result from mutations in ZIC3. Nature Genet. 17, 305– 308 (1997).
Kosaki, K. et al. Characterization and mutation analysis of human LEFTYA and LEFTYB, homologues of murine genes implicated in left-right axis development. Am. J. Hum. Genet. 64, 712–721 (1999).
Kosaki, R. et al. Left-right axis malformations associated with mutations in ACVR2B, the gene for human activin receptor type IIB. Am. J. Med. Genet. 82, 70–76 ( 1999).
Shen, M.M. & Schier, A.F. The EGF-CFC gene family in vertebrate development. Trends Genet . 16, 303–309 (2000).
Shen, M.M., Wang, H. & Leder, P. A differential display strategy identifies Cryptic, a novel EGF-related gene expressed in the axial and lateral mesoderm during mouse gastrulation. Development 124, 429 –442 (1997).
Zhang, J., Talbot, W.S. & Schier, A.F. Positional cloning identifies zebrafish one-eyed pinhead as a permissive EGF-related ligand required during gastrulation. Cell 92, 241–251 ( 1998).
Gritsman, K. et al. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97, 121– 137 (1999).
Gaio, U. et al. A role of the cryptic gene in the correct establishment of the left-right axis. Curr. Biol. 9, 1339– 1342 (1999).
Yan, Y.-T. et al. Conserved requirement for EGF-CFC genes in vertebrate left-right axis formation. Genes Dev. 13, 2527– 2537 (1999).
Minchiotti, G. et al. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mech. Dev. 90, 133–142 ( 2000).
Kane, D.A. et al. The zebrafish epiboly mutants. Development 123, 47–55 (1996).
Solnica-Krezel, L. et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 123, 67–80 (1996).
Osada, S.I. et al. Activin/nodal responsiveness and asymmetric expression of a Xenopus nodal-related gene converge on a FAST-regulated module in intron 1. Development 127, 2503– 2514 (2000).
Meno, C. et al. Mouse lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol. Cell 4, 287–298 (1999).
Niikawa, N., Kohsaka, S., Mizumoto, M., Hamada, I. & Kajii, T. Familial clustering of situs inversus totalis, and asplenia and polysplenia syndromes. Am. J. Med. Genet. 16, 43–47 ( 1983).
Saijoh, Y. et al. Left-right asymmetric expression of lefty2 and nodal is induced by a signaling pathway that includes the transcription factor FAST2. Mol. Cell 5, 35–47 ( 2000).
Collignon, D.B., Varlet, I. & Robertson, E. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature 381, 155–158 (1996).
Nomura, M. & Li, E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature 393, 786–790 (1998).
Acknowledgements
We thank the patients and their families for participation. R.D.B. is supported by a postdoctoral fellowship from the Damon Runyon-Walter Winchell Cancer Research Fund. A.F.S. is a Scholar of the McKnight Endowment Fund for Neuroscience. The authors are supported by grants from the NIH (B.C., A.F.S. and M.M.S.), the US Army Breast Cancer Research Program (M.M.S.) and the Division of Intramural Research, National Human Genome Research Institute, NIH (M.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bamford, R., Roessler, E., Burdine, R. et al. Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet 26, 365–369 (2000). https://doi.org/10.1038/81695
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81695
This article is cited by
-
De novo disruptive heterozygous MMP21 variants are potential predisposing genetic risk factors in Chinese Han heterotaxy children
Human Genomics (2022)
-
A novel nonsense PKD1L1 variant cause heterotaxy syndrome with congenital asplenia in a Han Chinese patient
Journal of Human Genetics (2022)
-
Delving into the Molecular World of Single Ventricle Congenital Heart Disease
Current Cardiology Reports (2022)
-
Discovery of a genetic module essential for assigning left–right asymmetry in humans and ancestral vertebrates
Nature Genetics (2022)
-
Genetics of Transposition of Great Arteries: Between Laterality Abnormality and Outflow Tract Defect
Journal of Cardiovascular Translational Research (2021)