Abstract
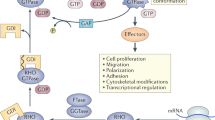
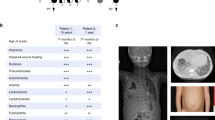
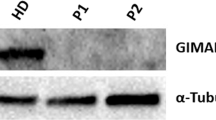
Griscelli syndrome (GS, MIM 214450), a rare, autosomal recessive disorder, results in pigmentary dilution of the skin and the hair, the presence of large clumps of pigment in hair shafts and an accumulation of melanosomes in melanocytes. Most patients also develop an uncontrolled T-lymphocyte and macrophage activation syndrome (known as haemophagocytic syndrome, HS), leading to death in the absence of bone-marrow transplantation1,2. In contrast, early in life some GS patients show a severe neurological impairment without apparent immune abnormalities3,4,5. We previously mapped the GS locus to chromosome 15q21 and found a mutation in a gene (MYO5A) encoding a molecular motor in two patients5. Further linkage analysis suggested a second gene associated with GS was in the same chromosomal region6. Homozygosity mapping in additional families narrowed the candidate region to a 3.1-cM interval between D15S1003 and D15S962. We detected mutations in RAB27A, which lies within this interval, in 16 patients with GS. Unlike MYO5A, the GTP-binding protein RAB27A appears to be involved in the control of the immune system, as all patients with RAB27A mutations, but none with the MYO5A mutation, developed HS. In addition, RAB27A-deficient T cells exhibited reduced cytotoxicity and cytolytic granule exocytosis, whereas MYO5A-defective T cells did not. RAB27A appears to be a key effector of cytotoxic granule exocytosis, a pathway essential for immune homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Griscelli, C. et al. A syndrome associating partial albinism and immunodeficiency. Am. J. Med. 65, 691–702 (1978).
Klein, C. et al. Partial albinism with immunodeficiency (Griscelli syndrome). J. Pediatr. 125, 886–895 (1994).
Hurvitz, H., Gillis, R., Klaus, S., Gross-Kieselstein, F. & Okon, E. A kindred with Griscelli disease: spectrum of neurological involvment. Eur. J. Pediatr. 152, 402–405 (1993).
Elejalde, B.R. et al. Mutations affecting pigmentation in man: I. neuroectodermal melanolysosomal disease. Am. J. Med. Genet. 3, 65–80 (1979).
Pastural, E. et al. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nature Genet. 16, 289–292 (1997).
Pastural, E. et al. Two genes are responsible for Griscelli syndrome at the same 15q21 locus. Genomics, 63, 299–306 (2000).
Tolmachova, T. et al. Cloning, mapping and characterization of the human RAB27A gene. Gene 239, 109–116 (1999).
Chen, D., Guo, J., Miki, T., Tachibana, M. & Gahl, W. Molecular cloning and characterization of Rab27a and Rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 60, 27–37 (1997).
Pfeffer, S.R. Rab GTPases: master regulators of membrane trafficking. Curr. Opin. Cell Biol. 6, 522–526 (1994).
Novick, P. & Zerial, M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496–504 (1997).
Kinsella, B. & Maltese, W. rab GTP-binding proteins with three different carboxyl-terminal cysteine motifs are modified in vivo by 20-carbon isoprenoids. J. Biol. Chem. 267, 3940–3945 (1992).
Chavrier, P. & Goud, B. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11, 466–475 (1999).
Stepp, S. et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286, 1957–1959 (1999).
Barrat, F.J. et al. Defective CTLA-4 cycling pathway in Chediak-Higashi syndrome: a possible mechanism for deregulation of T lymphocyte activation. Proc. Natl Acad. Sci. USA 96, 8645–8650 (1999).
Cheney, R.E. et al. Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell 75, 13–23 (1993).
Espreafico, E.M. et al. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J. Cell Biol. 119, 1541–1557 (1992).
Prekeris, R. & Terrian, D.M. Brain myosin V is a synaptic vesicle-associated motor protein: evidence for a Ca2+-dependent interaction with the synaptobrevin-synaptophysin complex. J. Cell Biol. 137, 1589–1601 (1997).
Echard, A. et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 279, 580–585 (1998).
Seabra, M.C., Ho, Y.K. & Anant, J.S. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J. Biol. Chem. 270, 24420–24427 (1995).
Stinchcombe, J.C. & Griffiths, G.M. Regulated secretion from hemopoietic cells. J. Cell Biol. 147, 1–5 (1999).
Dufourcq-Lagelouse, R. et al. Genetic basis of hemophagocytic lymphohistiocytosis syndrome. Int. J. Mol. Med. 4, 127–133 (1999).
Mazerolles, F. & Fischer, A. Binding of CD4 ligands induces tyrosine phosphorylation of phosphatidinylinositol-3 kinase p110 subunit. Int. Immunol. 10, 1897–1905 (1998).
Kim, S. & Yokoyama, W.M. NK cell granule exocytosis and cytokine production inhibited by Ly-49A engagement. Cell. Immunol. 183, 10–112 (1998).
Acknowledgements
We thank the patients and their families for participation; referring physicians; and C. Harré and N. Lambert for technical assistance. This work was supported by grants from INSERM, l'Association Française contre les Myopathies, l'Association de Recherche contre le Cancer and the BIOMED 2 Concerted Action PL 963007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ménasché, G., Pastural, E., Feldmann, J. et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25, 173–176 (2000). https://doi.org/10.1038/76024
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76024
This article is cited by
-
Griscelli syndrome type 1: a novel pathogenic variant, and review of literature
Molecular Genetics and Genomics (2023)
-
PMEL p.Leu18del dilutes coat color of Kumamoto sub-breed of Japanese Brown cattle
BMC Genomics (2022)
-
Development, characterization, and hematopoietic differentiation of Griscelli syndrome type 2 induced pluripotent stem cells
Stem Cell Research & Therapy (2021)
-
Successful rescue of a lethal Griscelli syndrome type 2 presenting with neurological involvement and hemophagocytic lymphohistiocytosis: a case report
BMC Pediatrics (2021)
-
Allogeneic hematopoietic stem cell transplant in rare hematologic disorders: a single center experience from Pakistan
Bone Marrow Transplantation (2021)