Abstract
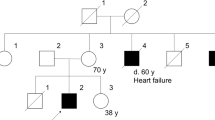
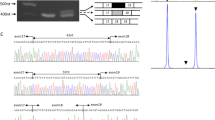
The lipodystrophies are a group of disorders characterized by the absence or reduction of subcutaneous adipose tissue. Partial lipodystrophy (PLD; MIM 151660) is an inherited condition in which a regional (trunk and limbs) loss of fat occurs during the peri-pubertal phase1,2. Additionally, variable degrees of resistance to insulin action, together with a hyperlipidaemic state, may occur and simulate the metabolic features commonly associated with predisposition to atherosclerotic disease3. The PLD locus has been mapped to chromosome 1q with no evidence of genetic heterogeneity4. We, and others, have refined the location to a 5.3-cM interval between markers D1S305 and D1S1600 (refs 5 , 6). Through a positional cloning approach we have identified five different missense mutations in LMNA among ten kindreds and three individuals with PLD. The protein product of LMNA is lamin A/C, which is a component of the nuclear envelope. Heterozygous mutations in LMNA have recently been identified in kindreds with the variant form of muscular dystrophy (MD) known as autosomal dominant Emery-Dreifuss MD (EDMD–AD; ref. 7) and dilated cardiomyopathy and conduction-system disease8 (CMD1A). As LMNA is ubiquitously expressed, the finding of site-specific amino acid substitutions in PLD, EDMD–AD and CMD1A reveals distinct functional domains of the lamin A/C protein required for the maintenance and integrity of different cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Köbberling, J. & Dunnigan, M. Familial partial lipodystrophy: two types of an X linked dominant syndrome, lethal in the hemizygous state. J. Med. Genet. 23, 120– 127 (1986).
Jackson, S., Howlett, T., McNally, P., O'Rahilly, S. & Trembath, R. Dunnigan-Köbberling syndrome: an autosomal dominant form of partial lipodystrophy. Q. J. Med. 90, 27–36 (1997).
Reaven, G. Role of insulin resistance in human disease. Diabetes 37, 1595–1607 (1988).
Peters, J. et al. Localisation of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21–22. Nature Genet. 18, 292–295 (1998).
Jackson, S. et al. A defect in the regional deposition of adipose tissue (partial lipodystrophy) is encoded by a gene at chromosome 1q. Am. J. Hum. Genet. 63, 534–540 ( 1998).
Anderson, J. et al. Confirmation of linkage of hereditary partial lipodystrophy to chromosome 1q21–22. Am. J. Med. Genet. 82, 161–165 (1999).
Bonne, G. et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nature Genet. 21 , 285–288 (1999).
Fatkin, D. et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 341, 1715–1724 (1999).
Garg, A., Peshock, R. & Fleckenstein, J. Adipose tissue distribution pattern in patients with familial partial lipodystrophy. J. Clin. Endocrinol. Metab. 84, 170–174 (1999).
Fisher, D.Z., Chaundhary, N. & Blobel, G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl Acad. Sci. USA 83, 6450– 6454 (1986).
McKeon, F.D., Kirschner, M.W. & Caput, D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319, 463–468 (1986).
Morris, G. & Manilal, S. Heart to heart: from nuclear proteins to Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 8, 1847–1851 (1999).
Stuurman, N., Heins, S. & Aebi, U. Nuclear lamins: their structure, assembly and interactions. J. Struct. Biol. 122, 42–66 (1998).
Wolin, S., Krohne, G. & Kirschner, M. A new lamin in Xenopus somatic tissues displays strong homology to human lamin A. EMBO J. 6, 3809–3818 (1987).
Sullivan, T. et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913–919 (1999).
Coyle, B. et al. Pendred syndrome (goitre and sensorineural hearing loss) maps to chromosome 7 in the region containing the nonsyndromic deafness gene DFNB4. Nature Genet. 12, 421– 423 (1996).
Shizuya, H. et al. Cloning and stable maintenance of 300-kilobase-pair fragments of human DNA in Escherichia coli using an F-factor-based vector. Proc. Natl Acad. Sci. USA 89, 8794– 8797 (1992).
Gregory, S., Howell, G.R. & Bentley, D.R. Genome mapping by fluorescent fingerprinting. Genome Res. 7, 1162–1168 (1997).
Lin, F. & Worman, H. Structural organisation of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, 16321–16326 ( 1993).
South, A. et al. Human epidermal differentiation complex in a single 2.5 Mbp long continuum of overlapping DNA cloned in bacteria integrating physical transcript maps. J. Invest. Dermatol . 112, 910– 918 (1999).
Cao, H. & Hegele, R. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partail lipodystrophy. Hum. Mol. Genet . 9, 109–112 (2000).
Acknowledgements
We thank the PLD patients and their relatives for support and encouragement, and R. Gwilliam for technical support. The following physicians provided clinical details and are responsible for the medical care of many of the patients described: P. Heyburn, R. Temple, R. Greenwood, P. McNally, T. Howlett, R. Corral, A. Johnson, J. Pinkney, J. Reckless and M. Dunnigan. This work had financial support from the British Diabetic Association (Ph.D. Studentship, D.J.L.) and the British Heart Foundation (project grant RCT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shackleton, S., Lloyd, D., Jackson, S. et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy . Nat Genet 24, 153–156 (2000). https://doi.org/10.1038/72807
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72807