Abstract
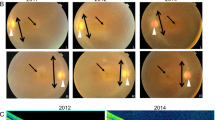
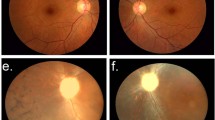
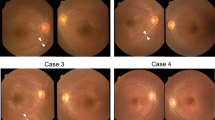
THE group of retinopathies termed retinitis pigmentosa (RP) greatly contribute to visual dysfunction in man with a frequency of roughly 1 in 4,000 (refs 1, 2). We mapped the first autosomal dominant RP (adRP) gene to chromosome 3q (refs 3, 4), close to the gene encoding rhodopsin, a rod photoreceptor pigment protein. Subsequently, mutations in this gene have been implicated as responsible for some forms of adRP5–9. Another adRP gene has been mapped to chromosome 8p (ref. 10). A third adRP gene in a large Irish pedigree has been mapped to chromosome 6p (refs 11,12), showing tight linkage with the gene for peripherin13,14, a photoreceptor cell-specific glycoprotein, which is thus a strong candidate for the defective gene. We have now identified a three-base-pair deletion which results in the loss of one of a pair of highly conserved cysteine residues in the predicted third transmem-brane domain of peripherin. This deletion segregates with the disease phenotype but is not present in unaffected controls, and suggests that mutant peripherin gives rise to retinitis pigmentosa.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bundey, S. & Crews, S. J. J. medl Genet. 21, 417–420 (1984).
Bunker, C. H. et al. Am. J. Opthalmol. 97, 357–365 (1984).
McWilliam, P. et al. Genomics 5, 619–622 (1989).
Farrar, G. J. et al. Genomics 8, 35–40 (1990).
Dryja, T. P. et al. Nature 343, 364–366 (1990).
Dryja, T. P. et al. New Engl. J. Med. 323, 1302–1307 (1990).
Inglehearn, C. F. et al. Am. J. hum. Genet. 48, 26–30 (1991).
Farrar, G. J. et al. Genomics (in the press).
Sung, C.-H. et al. Proc. natn. Acad. Sci. U.S.A. 88, 6481–6485 (1991).
Blanton, S. H. et al. Genomics (in the press).
Farrar, G. J. et al. Genomics (in the press).
Jordan, S. A. et al. Am. J. hum. Genet. (in the press).
Molday, R. S., Hicks, D. & Molday, L. Invest. Opthalmol. Vis. Sci. 28, 50 (1986).
Travis, G. H. et al. Genomics 10, 733–739 (1991).
Connell, G. & Molday, R. S. Biochemistry 29, 4691–4698 (1990).
Travis, G. H., Brennan, M. B., Danielson, P. E., Kozak, C. A. & Sutcliffe, J. G. Nature 338, 70–73 (1989).
Connell, G. et al. Proc. natn. Acad. Sci. U.S.A. 88, 723–726 (1991).
Orita, V., Suzuki, Y., Sekiya, T. & Hayashi, K. Genomics 5, 874–879 (1989).
Yandell, D. W. & Dryja, T. P. Am. J. hum. Genet. 45, 547–555 (1989).
Van Nie, R., Ivanyi, D. & Demant, P. Tissue Antigens 12, 106 (1978).
Sanyal, S. & Jansen, H. G. Neurosci. Lett. 21, 23 (1981).
Jansen, H. G. & Sanyal, S. J. comp. Neurol. 224, 71 (1984).
Travis, G. H., Sutcliffe, J. G. & Bok, D. Neuron 6, 61–70 (1991).
Flannery, J. G., Farber, D. B., Bird, A. C. & Bok, D. Invest. Ophthalmol. Vis. Sci. 30, 191–211 (1989).
Begy, C. & Bridges, C. D. Nucleic Acid Res. 18, 3058 (1990).
Connell, G., Molday, L. L., Reid, D. & Molday, R. S. in Retinal Degenerations (eds Hollyfield, J. G., Anderson, R. E. & LaVail, M. M.) 467–477 (CRC, Boca Raton, Florida, 1991).
Verlaan-de Vries, M. et al. Gene 50, 313–320 (1986).
Farr, C. J. et al. Proc. natn. Acad. Sci. U.S.A. 85, 1629–1633 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Farrar, G., Kenna, P., Jordan, S. et al. A three-base-pair deletion in the peripherin–RDS gene in one form of retinitis pigmentosa. Nature 354, 478–480 (1991). https://doi.org/10.1038/354478a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/354478a0
This article is cited by
-
Migration of pre-induced human peripheral blood mononuclear cells from the transplanted to contralateral eye in mice
Stem Cell Research & Therapy (2021)
-
PCR–SSCP: A Method for the Molecular Analysis of Genetic Diseases
Molecular Biotechnology (2008)
-
A novel mutation in Prph2, a gene regulated by Nr2e3, causes retinal degeneration and outer-segment defects similar to Nr2e3 rd7/rd7 retinas
Mammalian Genome (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.