Abstract
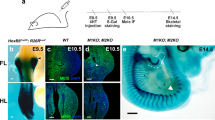
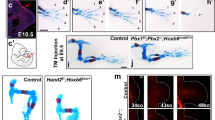
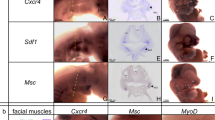
The skeletal muscles of the limbs develop from myogenic progenitors that originate in the paraxial mesoderm and migrate intothe limb-bud mesenchyme1. Among the genes known to be important for muscle development in mammalian embryos are those encoding the basic helix-loop-helix (bHLH) myogenic regulatory factors (MRFs; MyoD, Myf5, myogenin and MRF4)2,3,4 and Pax3, a paired-type homeobox gene that is critical for the development of limb musculature5,6,7. Mox1 and Mox2 are closely related homeobox genes that are expressed in overlapping patterns in the paraxial mesoderm and its derivatives8,9. Here we show that mice homozygous for a null mutation of Mox2 have a developmental defect of the limb musculature, characterized by an overall reduction in muscle mass and elimination of specific muscles. Mox2 is not needed for the migration of myogenic precursors into the limb bud, but it is essential for normal appendicular muscle formation and for the normal regulation of myogenic genes, as demonstrated by the downregulation of Pax3 and Myf5 but not MyoD in Mox2-deficient limb buds. Our findings show that the MOX2 homeoprotein is an important regulator of vertebrate limb myogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Christ, B., Jacob, H. J. & Jacob, M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat. Embryol. (Berl.) 150, 171–186 (1977).
Buckingham, M. Making muscle in mammals. Trends Genet. 8, 144–148 (1992).
Cossu, G., Tajbakhsh, S. & Buckingham, M. How is myogenesis initiated in the embryo? Trends Genet. 12, 218–223 (1996).
Rudnicki, M. A. & Jaenisch, R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays 17, 203–209 (1995).
Bober, E., Franz, T., Arnold, H. H., Gruss, P. & Tremblay, P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120, 603–612 (1994).
Goulding, M., Lumsden, A. & Paquette, A. J. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development 120, 957–971 (1994).
Williams, B. A. & Ordahl, C. P. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development 120, 785–796 (1994).
Candia, A. F. et al . Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development 116, 1123–1136 (1992).
Candia, A. F. & Wright, C. V. Differential localization of Mox-1 and Mox-2 proteins indicates distinct roles during development. Int. J. Dev. Biol. 40, 1179–1184 (1996).
Daston, G., Lamar, E., Olivier, M. & Goulding, M. Pax-3 is necessary for migration but not differentiation of limb muscle precursors in the mouse. Development 122, 1017–1027 (1996).
Deconinck, A. E. et al . Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell 90, 717–727 (1997).
Wachtler, F. & Jacob, M. Origin and development of the cranial skeletal muscles. Bibl. Anat. 29, 24–46 (1986).
Wright, W. E., Sassoon, D. A. & Lin, V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56, 607–617 (1989).
Sassoon, D. et al . Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature 341, 303–307 (1989).
Yee, S. P. & Rigby, P. W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 7, 1277–1289 (1993).
Bladt, F., Riethmacher, D., Isenmann, S., Aguzzi, A. & Birchmeier, C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376, 768–771 (1995).
Jagla, K. et al . Mouse Lbx1 and human LBX1 define a novel mammalian homeobox gene family related to the Drosophila ladybird genes. Mech. Dev. 53, 345–356 (1995).
Tajbakhsh, S., Rocancourt, D., Cossu, G. & Buckingham, M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89, 127–138 (1997).
Maina, F. et al . Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell 87, 531–542 (1996).
Kardon, G. Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019–4032 (1998).
Chevallier, A., Kieny, M. & Mauger, A. Limb-somite relationship: origin of the limb musculature. J.Embryol. Exp. Morphol. 41, 245–258 (1977).
Jacob, H. & Christ, B. in Teratology of the Limbs (eds Merker, H. J., Nau, H. & Neubert, D.) 89–97 (Walter de Gruyter and Co., Berlin, (1980).
Grim, M. & Wachtler, F. Muscle morphogenesis in the absence of myogenic cells. Anat. Embryol. (Berl.) 183, 67–70 (1991).
Yamamoto, M. et al . Coordinated expression of Hoxa-11 and Hoxa-13 during limb muscle patterning. Development 125, 1325–1335 (1998).
Mansour, S. L., Thomas, K. R. & Capecchi, M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336, 348–352 (1988).
Henrique, D. et al . Expression of a Delta homologue in prospective neurons in the chick. Nature 375, 787–790 (1995).
Acknowledgements
We thank S. Tajbakhsh for providing us with the Splotch2H embryos; R. Balling, P.Gruss and K. Jagla for providing us with Pax1, Pax3, and Lbx1 probes, respectively; T. Partridge for expert advice on histopathology; S. Hughes for useful discussions; H. Boyes for animal husbandry; and F.Costantini, in whose laboratory this work was initiated. This work was supported by the MRC.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 25 kb)
Rights and permissions
About this article
Cite this article
Mankoo, B., Collins, N., Ashby, P. et al. Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature 400, 69–73 (1999). https://doi.org/10.1038/21892
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/21892
This article is cited by
-
Identification of LINE retrotransposons and long non-coding RNAs expressed in the octopus brain
BMC Biology (2022)
-
A mosaic of conserved and novel modes of gene expression and morphogenesis in mesoderm and muscle formation of a larval bivalve
Organisms Diversity & Evolution (2022)
-
A genome-wide scan to identify signatures of selection in two Iranian indigenous chicken ecotypes
Genetics Selection Evolution (2021)
-
HES and Mox genes are expressed during early mesoderm formation in a mollusk with putative ancestral features
Scientific Reports (2021)
-
Understanding paraxial mesoderm development and sclerotome specification for skeletal repair
Experimental & Molecular Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.