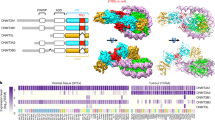
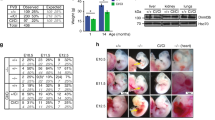
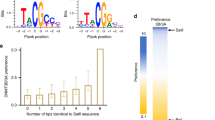
Abstract
DNA-methylation patterns are important for regulating genome functions, and are determined by the enzymatic processes of methylation and demethylation. The demethylating enzyme has now been identified: a mammalian complementary DNA encodes a methyl-CpG-binding domain, bears a demethylase activity that transforms methylated cytosine bases to cytosine, and demethylates a plasmid when the cDNA is translated or transiently transfected into human embryonal kidney cells in vitro. The discovery of this DNA demethylase should provide a basis for the molecular and developmental analysis of the role of DNA methylation and demethylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegfried, Z. & Cedar, H. DNA methylation: a molecular lock. Curr. Biol. 7, 305–307 (1997).
Nan, X., Campoy, F. J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471–481 (1997).
Nan, X. S. et al. Transcriptional repression by the methyl-CpG-binding protein MECP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Jones, P. L. et al. Methylated DNA and MECP2 recruit histone deacetylase to repress transcription. Nature Genet. 19, 187–191 (1998).
Li, E., Bestor, T. H. & Jaenish, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Razin, A. & Riggs, A. D. DNA methylation and gene function. Science 210, 604–610 (1980).
Brandeis, M., Ariel, M. & Cedar, H. Dynamics of DNA methylation during development. Bioassays 15, 709–713 (1993).
Kafri, T., Gao, X. & Razin, A. Methanistic aspects of genome-wide demethylation in the preimplantation mouse embryo. Proc. Natl Acad. Sci. USA 90, 10558–10562 (1993).
Monk, M., Boubelik, M. & Lehnert, S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 99, 371–382 (1987).
Bestor, T., Laudano, A., Mattaliano, R. & Ingram, V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J. Mol. Biol. 203, 971–983 (1988).
Bestor, T. H. & Verdine, G. L. DNA methyltransferases. Curr. Opin. Cell Biol. 6, 380–389 (1994).
Razin, A. et al. Replacement of 5-methylcytosine by cytosine: a possible mechanism for transient DNA demethylation during differentiation. Proc. Natl Acad. Sci. USA 83, 2827–2831 (1986).
Vairapandi, M. & Duker, N. J. Enzymic removal of 5-methylcytosine from DNA by a human glycosylase. Nucleic Acids Res. 21, 5323–5327 (1993).
Jost, J. P., Siegmann, M., Sun, L. J. & Leung, R. Mechanisms of demethylation in chicken embryos—purification and properties of a 5-methylcytosine-DNA glycosylase. J. Biol. Chem. 270, 9734–9739 (1995).
Weiss, A., Keshet, I., Razin, A. & Cedar, H. DNA demethylation in vitro: involvement of RNA. Cell 86, 709–718 (1996).
Cross, S. H., Meehan, R. R., Nan, X. & Bird, A. Acomponent of the transcriptional repressor MeCP1 shares a motif with DNA methyltransferase and HRX proteins. Nature Genet. 16, 256–259 (1997).
Boguski, M. S., Lowe, T. M. & Tolstoshev, C. M. dbEST-database for expressed sequence tags. Nature Genet. 4, 332–373 (1995).
Henrich, B. & Bird, A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 18, 6538–6547 (1998).
Corpet, F., Gouzy, J. & Kahn, D. The ProDom database of protein domain families. Nucleic Acids Res. 26, 323–326 (1998).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Bairoch, A., Bucher, P. & Hofman, K. The PROSITE database, its status in 1997. Nucleic Acids Res. 25, 217–221 (1997).
Rost, B. PHD: predicting one-dimensional protein structure by profile based neural networks. Methods Enzymol. 266, 525–539 (1996).
Lupas, A., Van Dyke, M. & Stock, J. Predicting coiled coils from protein sequences. Science 252, 1162–1164 (1991).
O'Shea, E. K., Rutkowski, R. & Kim, P. S. Evidence that the leucine zipper is a coiled coil. Science 243, 538–542 (1989).
Nur, I. et al. Prokayotic and eukaryotic traits of DNA methylation in spiroplasmas (mycoplasmas). J. Bacteriol. 164, 19–24 (1985).
Szyf, M., Theberge, J. & Bozovic, V. Ras induces a general DNA demethylation activity in mouse embryonal P19 cells. J. Biol. Chem. 270, 12690–12696 (1995).
Szyf, M. DNA methylation properties: consequences for pharmacoloty. Trends Pharmacol. Sci. 7, 233–238 (1994).
Swisher, J. A., Rand, E., Cedar, H. & Pyle, A. M. Analysis of putative RNase sensitivity and protease insensitivity of demethylation activity in extracts from rat myoblasts. Nucleic Acids Res. 26, 5573–5580 (1998).
Acknowledgements
We thank B. ten Oever and H.-Y. Shiu for technical assistance and O. A. Mamer for help with mass spectrometry. This research was supported by the National Cancer Institute of Canada.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhattacharya, S., Ramchandani, S., Cervoni, N. et al. A mammalian protein with specific demethylase activity for mCpG DNA. Nature 397, 579–583 (1999). https://doi.org/10.1038/17533
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/17533
This article is cited by
-
Induction of CD4+CD25+Foxp3+ regulatory T cells by mesenchymal stem cells is associated with RUNX complex factors
Immunologic Research (2018)
-
Epigenetic modifications of gene expression by lifestyle and environment
Archives of Pharmacal Research (2017)
-
Epigenetic Features Induced by Ischemia-Hypoxia in Cultured Rat Astrocytes
Molecular Neurobiology (2016)
-
The dynamic epigenome and its implications for behavioral interventions: a role for epigenetics to inform disorder prevention and health promotion
Translational Behavioral Medicine (2016)
-
Prospects for the development of epigenetic drugs for CNS conditions
Nature Reviews Drug Discovery (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.