Abstract
The Bardet–Biedl syndrome (BBS) is a significant genetic cause of chronic and end-stage renal failure in children. Despite being a relatively rare recessive condition, BBS has come to prominence during the past few years owing to revelations of primary cilia dysfunction underlying pathogenesis. The study of this multi-system disorder, which includes obesity, cognitive impairment, genito-urinary tract malformations and limb deformities, is beginning to reveal insights into several aspects of mammalian development and organogenesis. Involvement of BBS proteins in disparate pathways such as the non-canonical Wnt and Sonic Hedgehog pathways is highlighting their interplay in disease pathogenesis. Here we review the recent developments in this emerging field, with the emphasis on the renal component of the syndrome and potential future directions.
Similar content being viewed by others
Overview of Bardet–Biedl syndrome
The unlikely ensemble of signs that the Bardet–Biedl syndrome presents has bewildered doctors for over a hundred years. In 1865 Laurence and Moon reported the first case of an obese, visually impaired girl with intellectual disabilities [1]. This triad of features was extended in the 1920s by two independent reports by George Bardet and Artur Biedl describing the additional characteristics of polydactyly and hypogenitalism [2, 3]. These remain the cardinal features of Bardet–Biedl syndrome (BBS), but further manifestations of the disease have since been recognised. Polycystic kidneys are the most likely cause of premature death from the syndrome, combined with complications caused by overweight, including type II diabetes, hypertension and hypercholesterolaemia [4] (see Table 1 and Fig. 2 for diagnostic criteria). This review summarises some key recent findings in BBS research, focusing on the renal components of the syndrome. In the light of recent revelations we speculate on the aetiology of several aspects of the syndrome that may lie beyond the cilium.
Putative pathomechanism for renal cystic hyperplasia in BBS. Top left Urine flow through the kidney tubule causes an influx of Ca2+ ions through polycystin 2 (PC2), while polycystin 1 (PC1) anchors the transcriptional complex of P100 and STAT6 in the cilium. Concurrently, inversin is translocated to the nucleus, where it targets cytoplasmic Dishevelled for destruction, activating the non-canonical pathway and causing cells to differentiate. The ciliary localisation of PC1/2 and inversin may be dependent on BBS proteins. Top right Absence of urine flow reduces Ca2+ influx and causes release of P100 and STAT6, allowing them to enter the nucleus to activate transcription. It also prevents translocation of inversin to the cytoplasm, maintaining the cytoplasmic pool of Dishevelled, which activates the canonical Wnt pathway and causes proliferation. Bottom left A lack of BBS protein function may inhibit proper transport of PC1/2 to the distal tip of the cilium and also prevent translocation of inversin to the cytoplasm. This would cause inappropriate activation of STAT6 target and maintenance of cytoplasmic Dishevelled, leading to unregulated cell proliferation. Additionally, lack of BBS protein function could disturb planar cell polarity (PCP). The combination of disorganised cell polarity and cell division could cause the various abnormalities seen in BBS
Although BBS is considered to be a developmental disorder, the only clues in utero may be hexadactyly and hyperechoic kidneys [7]. Birth weight tends to be normal, but rapid weight gain begins after the first year, probably due to hyperphagia rather than metabolic abnormalities [8]. Language acquisition may be delayed until the child is 4 years of age. Diagnosis of BBS is often only established once the vision begins to degrade. Night blindness manifests when the child is around 8 years and is followed by loss of peripheral vision, usually progressing to significant blindness by 15 years [4].
Twelve BBS genes identified
Early linkage studies revealed a high level of heterogeneity, culminating in the discovery of not one but, so far, 12 genes (summarised in Table 2). When mutated, any of these genes can cause the plethora of BBS features. As there appears to be no correlation between genotype and phenotype, it has been hypothesised that the different BBS proteins are functioning in a common cellular process [9]. In 2003 Ansley et al. proposed that BBS was caused by a dysfunction of the cilium and its associated basal body, an organelle derived from the centriole acting as a nucleation site for ciliary axonemal microtubules [10]. They discovered BBS8 and showed that its protein localises to the centrosome and basal body. BBS8 interacts with peri-centriolar material 1 (PCM1), a protein important for ciliogenesis. Further, in the nematode C. elegans, bbs8 is regulated by the transcription factor daf-19 (an RFX-type transcription factor), a regulator of genes involved in ciliogenesis and transport [10].
Since this initial association of BBS8 with the basal body, putative roles for other BBS proteins are being established. These are summarised in Table 2. BBS1, 2, 4, 5, 6, and 8 all localise to the basal body and peri-centriolar region. In C. elegans BBS7 and BBS8 localise to the base of the cilium and are transported along the axoneme of sensory neurons [17]. BBS4 functions in the microtubule apparatus and interacts with subunits of dynactin, implying a role in retrograde intraflagellar transport (IFT) [14]. BBS6, 10, and 12 are likely chaperonins, which may facilitate protein folding and together account for one third of all cases [21]. Why mutations in chaperonins should cause the features of BBS is, as yet, unknown, but it could be due to aberrant folding of proteins essential for IFT or ciliogenesis.
Aetiology lies in the cilium
Cilia are ancient eukaryotic organelles that project from the cell surface. They fall into two classes: motile and immotile. Motile cilia consist of an outer ring of nine doublets of microtubules surrounding an inner pair—the “9 + 2” arrangement. Radial spokes link the outer ring doublets to the inner doublet, and dynein motor proteins, spanning adjacent outer doublets, power the beating of the cilium. This beating motion is used to drive fluid flow in the respiratory tract and oviduct, and propels spermatozoa.
The features of BBS do not, in the main, appear to reflect a defect in motile cilia (except for aflagellate spermatozoa). Rather, it seems that the immotile sensory (also called primary) cilia are predominantly affected. These consist of a “9 + 0” arrangement of microtubule doublets and lack the central microtubule pair present in motile cilia. Until recently, primary cilia were considered to be evolutionary vestiges, with no obvious function. Now their role in vertebrate physiology is finally being elucidated; many key signalling pathways rely on the cilium for their function. The list of molecular pathways with key components localising to the primary cilium includes: PDGFRα growth factor signalling [22], hedgehog signalling [23], epidermal growth factor signalling [24], and 5-HT6 serotonin signalling [25]. See Marshall and Nonaka (2006) for a more comprehensive list of cilia-dependent signalling pathways [26]. The study of BBS is improving our understanding of the importance and complexity of these organelles in development and homoeostasis.
It is now thought that BBS proteins participate in IFT [17, 27]. Because cilia lack ribosomes, all proteins involved in their construction and function must first be imported and then shuttled along the ciliary axoneme by a raft of proteins [9]. Cargo bound for the distal end of the cilium is loaded in the cytoplasm at the transition zone at the base of the cilium. Anterograde transport is facilitated by the microtubule motor protein kinesin-2. Retrograde transport back to the cytoplasm deploys the dynein–dynactin motor complex. See Blacque and Leroux for a detailed review of the roles of BBS proteins in IFT [28].
Some BBS proteins are thought to function as adaptors, helping to load cargo proteins at the proximal cytoplasmic end for carriage to the distal tip [17]. BBS proteins are also thought to be required for retrograde transport, where proteins are shuttled back to the cytoplasm for recycling. Figure 1 shows how the IFT processes are coordinated in the cilium. Mutations in a BBS gene reduce or abolish their protein function, disrupting IFT. This has been demonstrated in C. elegans, where abrogation of bbs7 or bbs8 both reduces the length of cilia in neurons (structure) and disrupts IFT machinery, leading to improper transport of kinesin and other IFT components along the cilium (function) [17].
Because BBS proteins are not absolutely required for ciliary function, mutations in BBS genes do not cause as dramatic an effect as mutations in core IFT proteins such as Kif3a, which die at 10 days after fertilisation [29]. As such, the clinical phenotypes of BBS mutants are less severe than one might expect if IFT were completely abrogated. Some BBS features can be readily attributed to deficient IFT: retinitis pigmentosa (RP) can result from transport defects in the connecting cilium of the rod and cone cells in the retina, leading to progressive degeneration of the photoreceptors [30]. Figure 2 summarises these features. Anosmia is common amongst patients, owing to flaws in olfactory cilia [11]. Infertility has been reported in Bbs null mice, where immotility was attributed to failure of the spermatozoa flagella to develop [31]. Some BBS patients have situs inversus, a complete reversal of visceral laterality, arising from defective cilia in the early embryonic node. Perhaps the best characterised feature of a presumed ‘ciliopathy’ is the cystic kidneys, with their aetiology in aberrant ciliary function.
Renal disease is a major cause of mortality in BBS
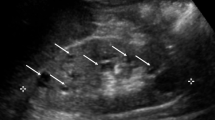
Beales et al. (1999) reported imaging 59 BBS patients and found that 46% had abnormal renal structure but only 5% had functional impairment at the time of scanning [4]. Large, hyperechoic kidneys can be detected prenatally by ultrasound but are not necessarily a predictor of subsequent dysfunction [7]. Examples of renal histopathology observed in BBS patients are shown in Fig. 3.
Examples of renal histopathology in BBS patients. a, b Low and high power micrographs showing tubular dilatation in a biopsy from a BBS patient’s kidney (Histopathological sections courtesy of Dr. Neil Sebire, Great Ormond Street Hospital). c Fundoscopy showing retinitis pigmentosa with cataract. d Abdominal CT scan documenting cystic kidneys (arrowed). e, f Post-axial polydactyly in a hand and foot from the same child with BBS
Two reports in the 1970s described seven patients with cystic spaces communicating with collecting ducts [32, 33]. O’Dea (1996) conducted a prospective cohort study on 38 patients in Newfoundland, where the disease is ten-times more prevalent than in mainland Europe or America due to founder effects [34]. They found that 96% of patients had foetal lobulation, calyceal cysts, diverticula, or clubbing. Twenty-five percent of patients suffered functional impairment, fatally in two patients with end-stage renal disease. Another patient died from metastatic renal cancer.
Beales and colleagues documented an excess of relatively early-onset renal cell carcinomas in obligate carriers of BBS mutations. Furthermore, they detected loss of heterozygosity in tumour sections at several BBS loci [35]. Ersoy et al. (2005) reported a 20-year-old patient who had undergone renal transplantation who then developed primary central nervous system lymphoma. They suggested that BBS patients should be carefully monitored for developing malignancies [36].
In a cohort of 20 patients in Newfoundland, Harnett and colleagues identified three with end-stage renal failure [37]. Fourteen (70%) patients could not concentrate urine above 750 mosmol/kg body weight, even after vasopressin treatment. Upon ammonium chloride administration, urinary pH fell in only 13 patients (65%). Structural abnormalities tend to be common, with varying degrees of functional impairment. Alton and McDonald asserted that 30% of BBS patients die from uraemia [32]. Chronic renal dialysis, or transplantation, has been the only successful mode of managing these problems for most patients. Langer and colleagues (2005) presented details of a 57-year-old patient who had undergone cadaveric kidney transplantation. Despite developing subsequent pneumonia, cytomegalovirus and scabies infections post-operatively, he was fully rehabilitated within 18 months [38].
Currently, in our own cohort, approximately 10% of BBS patients have undergone transplantation (unpublished data). An analysis of the long-term outcome of renal transplantation in BBS is underway, but initial observations suggest that children manage very well in the immediate-to-medium post-operative period (unpublished data). Modern steroid-sparing, anti-rejection regimens should be implemented to optimise glycaemic control and avoid exacerbation of obesity.
Ciliary origins of kidney disease in BBS
Renal abnormality in BBS is thought to arise as a direct result of compromised cilia function, although this has yet to be proven. The origin of this hypothesis lies in the oak ridge polycystic kidney disease (orpkd) mouse mutant, which has dilated proximal tubules and cysts, and its cilia are short and poorly formed [39]. The mutation was mapped to the gene encoding polaris, a protein required for assembly of renal cilia [40]. Mutations in the PKHD1 gene cause autosomal recessive polycystic kidney disease (ARPKD). The protein product, polyductin, co-localises with polycystin 2 at the base of the cilium [41].
In the nephron, cilia projecting into the lumen from the apical surface of epithelial cells bend in response to urine flow. The degree of bending is directly related to the fluid pressure and causes the opening of calcium channels in the cilium. The influx of calcium ions is proportional to the flow and activates intracellular signalling pathways thought to regulate the cell’s decision either to proliferate or differentiate. These calcium channels are formed by a complex of polycystin 1 and polycystin 2 (PC1 and PC2), encoded by the PKD1 and PKD2 genes, respectively, which cause autosomal dominant polycystic kidney disease (ADPKD) when mutated [42]. PC1 binds to the transcription factor STAT6 and its co-factor p100. Upon mechanical shearing caused by urine flow, PC1 disassociates from STAT6, which travels down the cilium to enter the nucleus where it activates target genes [43]. It remains to be discovered whether BBS proteins are involved in the transport and/or localisation of these ciliary proteins.
Wnt signalling during kidney development may be regulated at the cilium
Recent evidence is emerging of additional regulatory roles for the cilium. It seems that the cilium may provide a regulatory switch between the canonical and non-canonical Wnt pathways [44]. In the Wnt pathway, diffusible extra-cellular Wnt ligands bind to their respective membrane-bound Frizzled receptors, leading to the activation of intracellular Dishevelled. If this then activates β-catenin, the canonical pathway is effected and is associated with re-entry into the cell cycle and proliferation. This pathway is required for branching morphogenesis during embryonic kidney development. Canonical Wnt signalling is dysregulated in Invs mice kidneys, which have a mutation in the gene encoding the ciliary protein inversin. In humans this gene (NPHP2) causes nephronophthisis (the most common autosomal recessive cause of early-onset renal failure) type II when mutated, which comprises interstitial cell infiltration, with fibrosis, and duct proliferation with cyst development [45]. Interestingly, the kidneys are massively enlarged in NPHP2 mutated mice and patients. Inversin is activated by tubular fluid flow and targets Dishevelled for destruction, inhibiting the canonical Wnt pathway and driving cells down the non-canonical Wnt or PCP pathway towards differentiation and polarisation throughout the plane of the tubular epithelium.
In support of this hypothesis, we demonstrated that BBS proteins are involved in the PCP pathway. Of Bbs4 null mice, 14% display anterior neural tube defects indicative of PCP dysfunction [30]. In adulthood, null mice have disorientated stereociliary bundles on the outer hair cells of the cochlea, and mice heterozygous for both Looptail (also known as Vangl2, a “core” PCP gene) and bbs4 have phenotypes reminiscent of PCP mutants, whilst both single heterozygotes are normal. Confirmation of the interaction was made in zebrafish by the injection of a bbs4 morpholino on a PCP mutant background and observing enhancement of the PCP phenotype.
A subsequent study by Fischer and colleagues suggested that this interaction may have implications for BBS-related cystogenesis. They measured the mitotic orientation of cells taken from the kidney tubules of a rat model of polycystic kidney disease and found them to be significantly disorientated [46]. They attributed this to mis-expression of polyductin (also called fibrocystin), a ciliary membrane protein mutated in ARPKD. As mitotic spindle orientation is regulated by the PCP pathway, aberrations caused by BBS protein dysfunction could cause similar mitotic defects in BBS kidneys. These results point to a need for hyperplasia caused by overactivity of the canonical Wnt pathway, combined with concomitant defects in planar cell polarisation.
BBS proteins in renal pathogenesis
So far, the precise role of BBS proteins in renal pathogenesis is unclear. It is possible that BBS proteins are involved in the transport of proteins such as the polycystin ion channels and polaris to the distal end of the cilium, where they function as components of the sensory apparatus.
It is also possible that BBS proteins are somehow regulating the function of inversin, which, in turn, is involved in the selection of canonical versus non-canonical Wnt pathways. If BBS proteins are absent, inversin function may be compromised, with the balance shifted in favour of the canonical pathway and promoting cystic hyperplasia. Disrupted PCP activity could be associated with a disordered renal epithelium. This, combined with a concomitant boost to canonical Wnt signalling, might explain why some BBS patients show complex renal involvement; such as mesangial cell proliferation, cystic dilation of tubules, and corticomedullary cysts [47].
The ciliary plexus
It is emerging that the cilium is indispensable for certain key developmental signalling cascades. It is now becoming clear that many discrete pathways converge at, or are regulated by, the cilium. Park et al. recently showed that defects in the PCP proteins Inturned and Fuzzy cause defects in ciliogenesis, with concomitant effects on Sonic Hedgehog (Shh) signalling [48].
Another ciliopathy, oro-facial digital type I (OFD1), shares some cardinal features with BBS, notably cystic kidneys, polydactyly and craniofacial dysmorphology. Ferrante et al. (2006) showed that when mutated this protein causes misregulation of downstream Shh pathway targets in the neural tube [49]. In the developing limb the expression patterns of Gli3 and Patched1, direct Shh targets are unaltered; whilst various Hox genes essential for digital patterning are disrupted. This does not indicate that Gli3 function is unimpaired—Shh patterning depends on the balance between Gli3’s active and repressive forms, not the overall level of Gli3 transcript. Several other studies have shown that defects in ciliary transport cause an imbalance between active and repressive forms of Gli3, particularly when exposed to Shh protein [50–52].
BBS patients have several hallmarks of defective Shh signalling, polydactyly being the most obvious amongst them. Additionally, many patients have agenesis or hypoplasia of the corpus callosum, and a single central incisor, both elements of the holoprosencephaly spectrum (personal observations). It is perplexing why ciliopathies such as BBS, Meckel syndrome, and OFD show features of sonic abnormalities, whilst others, such as polycystic kidney disease (PKD) and Senior–Loken syndrome (nephronophthisis with retinal degeneration) do not.
Support for the involvement of the cilium in regulating both Shh and PCP pathways in development was recently demonstrated by Park and colleagues [48]. It was also recently shown that the Gli transcription factors which act downstream of Shh localise to the cilium too, and require IFT for their processing and transcriptional activity [23]. Interruption of the activity of Gli3 represses the activation of target genes responsible for renal morphogenesis (such as Pax2 and Sall1) and results in decreased branching morphogenesis and dilated tubules [53]. Together, these observations may help account for some of the renal tissue abnormalities in BBS.
If BBS proteins are essential for all the above-mentioned signalling pathways and ciliary processes, one would expect the clinical phenotype to represent full loss of all involved molecules. However, BBS proteins implicated in IFT seem to be partially dispensable. In C. elegans, knock-out of BBS7 and BBS8 destabilises transport of IFT complexes but does not abrogate function altogether [54]. Using microtubule gliding assays, Pan et al. recently proposed that BBS7 and BBS8 act as a molecular bridge, holding the IFT particle complexes A and B together [55]. This holding force seems to be necessary to coordinate transport along the distal-most portion of the cilium. A lack of BBS protein function could result in disorganised shuttling of proteins whose distal localisation in the cilium is critical for proper function. Proteins transported in this way may include the polycystins and sonic hedgehog pathway components such as smoothened and Gli.
Not all cardinal features can be attributed to the cilium
Ciliary dysfunction currently fails to explain, directly, hypogenitalism, polydactyly, mental retardation, or obesity in BBS. Nonetheless, we can postulate several mechanisms. For example, development of the genitalia and gonads is partly controlled by Shh and bone morphogenetic protein 4 (BMP4) [56]. It is possible that misregulation of the Shh pathway is partly responsible, in combination with endocrine malfunction.
Post-axial polydactyly is present in 70% of patients. It is known that cells of the developing limb bud, in both ectoderm and mesenchyme, are ciliated [23]. Along with the known reliance of the Shh pathway on cilia, it is tempting to speculate that aberrant Shh signalling in the limb bud is the cause of the limb deformities seen in BBS. Post-axial polydactyly is also a primary feature of Pallister–Hall syndrome, a condition caused by a mutation in Gli3 [57].
The aetiology of obesity in BBS is more problematic although we do know that patients’ resting metabolic rate is normal [8]. Anecdotal reports hint that appetites are difficult to satiate, implicating a defect in the satiety centre of the hypothalamus. Like IFT, fast-axonal transport (FAT) is microtubular based and relies on dynein motors [58]. BBS proteins could play a part in transducing information about the satiety level to the neuronal cell bodies. However, there are no published data supporting a role for BBS proteins in FAT—as such, this is highly speculative. In C. elegans it has been shown that BBS proteins function in ciliated neurons that sense external and internal nutrient levels. bbs1 mutants act synergistically to enhance accumulation of lipids in a C. elegans mutant with impaired β-oxidation of fatty acids [59].
Despite progress in the identification of pathways affected in BBS, it is proving difficult to recognise the point of interaction and, in particular, the components at fault. Nonetheless, it will be important to determine these factors, as they may reveal therapeutic targets. For example, two other centrosomally localised proteins—tuberose sclerosis 1 and tuberose sclerosis 2 (TSC1 and TSC2)—cause renal cysts when their genes are mutated. As both proteins inhibit mTOR, a kinase controlling cell proliferation, it is possible to reduce cyst growth by treatment with the mTOR inhibitor rapamycin. Furthermore, it has been shown that the mTOR pathway is regulated by polycystin 1 and that ADPKD patients’ cysts can be ameliorated by rapamycin treatment [60]. Determining if BBS proteins impinge on these pathways in the kidney may provide a remedy that is already well characterised.
Beyond the cilium?
Unexpected new BBS genes (BBS9-12) are being discovered that do not show any intuitive relationship with the cilium or IFT. It is, therefore, likely that BBS proteins have extra-ciliary roles. Recently identified BBS genes include BBS9 (also called B1), a parathyroid hormone-responsive protein [18]. BBS10 and BBS12 show similarity to BBS6, and are likely to encode chaperonins with the potential to fold nascent proteins. BBS10 is a major pathogenic locus, accounting for 20% of cases [19]. It has been suggested that BBS6, BBS10, and BBS12 may act redundantly [21].
BBS11 (also called TRIM32) mutations have been identified only in a single consanguineous Bedouin family [20]. It encodes an E3 ubiquitin ligase and may be involved in protein degradation. Interestingly, TRIM32 has recently been shown to interact with a protein important for the sumoylation pathway; Piasy [61]. Whilst ubiquitination degrades proteins, sumoylation stabilises them. BBS11 may be involved in the regulation of the stability and lifetime of proteins associated with the basal body and IFT. Subcellular localisation for these newly discovered proteins is unconfirmed at present, and their function in wild type organisms has yet to be elucidated.
So far, we know that the PCP, the Shh, and, possibly, the canonical Wnt (via inversin) pathways rely, at least in part, on the cilium for their function and are intersected by the BBS proteins. The future direction of research in this field will involve the precise definition of the molecules that are being regulated by BBS protein malfunction, how this impacts on their downstream effectors, and whether this knowledge could lead to any therapeutic intervention.
We must determine why BBS proteins with different functions produce a syndrome with such a consistent phenotype. Conversely, why is it that individuals with the same mutations display such variable phenotypes? Steps towards answering this question were made by the recent discovery of a novel gene, MGC1203, which contributes epistatically to BBS mutations and can enhance the phenotype, at least in zebrafish embryos [62]. The combined input of epistatic and mechanistic studies, derived from multiple animal models, should bring us closer to understanding this complex disease.
Conclusion
BBS is a rare pleiotropic syndrome, the study of which has been both challenging and informative well beyond the scope of the disease itself. So far, the cilium has been held responsible for the bulk of the pathology, but the precise mechanisms and pathways involved are only just being revealed. The PCP and, potentially, sonic hedgehog pathways may contribute, in some part, to our understanding of these problems. The biology of polycystic kidney disease is in accord with our current understanding of BBS and may explain the associated renal dysgenesis. In turn, this is likely to involve imbalanced Wnt signalling, but further direct functional evidence is required.
References
Laurence JZ, Moon RC (1866) Four cases of retinitis pigmentosa occurring in the same family accompanied by general imperfection of development. Ophthalmol Rev 2:32–41
Bardet G (1920) Sur un syndrome d’obesite congenitale avec polydactylie et retinite pigmentaire (contribution a l’etude des formes cliniques de l’obesite hypophysaire) University of Paris
Biedl A (1922) Ein Geschwisterpaar mit adiposo-genitaler Dystrophie. Dtsch Med Wochenschr 48:1633
Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA (1999) New criteria for improved diagnosis of Bardet–Biedl syndrome: results of a population survey. J Med Genet 36(6):437–446
Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS (2005) Clinical and genetic epidemiology of Bardet–Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A 132(4):352–360
Lorda-Sanchez I, Ayuso C, Ibanez A (2000) Situs inversus and Hirschprung disease: two uncommon manifestations in Bardet–Biedl syndrome. Am J Med Genet 90(1):80–81
Cassart M, Eurin D, Didier F, Guibaud L, Avni EF (2004) Antenatal renal sonographic anomalies and postnatal follow-up of renal involvement in Bardet–Biedl syndrome. Ultrasound Obstet Gynecol 24(1):51–54
Grace C, Beales P, Summerbell C, Jebb SA, Wright A, Parker D, Kopelman P (2003) Energy metabolism in Bardet–Biedl syndrome. Int J Obes Relat Metab Disord 27(11):1319–1324
Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3(11):813–825
Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425(6958):628–633
Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N (2004) Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet 36(9):994–998
Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC (2004) Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA 101(47):16588–16593
Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, Green JS, Lewis RA, van Haelst MM, Parfrey PS, Baillie DL, Beales PL, Katsanis N, Davidson WS, Leroux MR (2004) Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet–Biedl syndrome. Nat Genet 36(9):989–993
Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL (2004) The Bardet–Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet 36(5):462–470
Li JB, Gerdes JM, Haycraft C, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC (2004) Comparative genomics identifies a flagellar and basal body proteome that Includes the BBS5 human disease gene. Cell 117(4):541–552
Kim JC, Ou YY, Badano JL, Esmail MA, Leitch CC, Fiedrich E, Beales PL, Archibald JM, Katsanis N, Rattner JB, Leroux MR (2005) MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet–Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci 118(5):1007–1020
Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RHA, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR (2004) Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev 18(13):1630–1642
Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, Sheffield VC (2005) Comparative genomics and gene expression analysis identifies BBS9, a new Bardet–Biedl syndrome gene. Am J Hum Genet 77(6):1021–1033
Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, Jalk N, Vicaire S, Sarda P, Hamel C, Lacombe D, Holder M, Odent S, Holder S, Brooks AS, Elcioglu NH, Silva ED, Rossillion B, Sigaudy S, de Ravel TJ, Lewis RA, Leheup B, Verloes A, Amati-Bonneau P, Megarbane A, Poch O, Bonneau D, Beales PL, Mandel JL, Katsanis N, Dollfus H (2006) BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet 38(5):521–524
Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KY, Huang J, Elbedour K, Carmi R, Slusarski DC, Casavant TL, Stone EM, Sheffield VC (2006) Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet–Biedl syndrome gene (BBS11). Proc Natl Acad Sci USA 103(16):6287–6292
Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, Hamel C, de Ravel TJ, Lewis RA, Friederich E, Thibault C, Danse JM, Verloes A, Bonneau D, Katsanis N, Poch O, Mandel JL, Dollfus H (2007) Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet–Biedl syndrome. Am J Hum Genet 80(1):1–11
Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST (2005) PDGFRα signaling Is regulated through the primary cilium in fibroblasts. Curr Biol 15(20):1861–1866, 25
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. Plos Genet 1(4):e53
Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L (2005) PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25(18):8285–8298
Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D (2000) Localization of 5-HT6 receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 872(1–2):271–275
Marshall WF, Nonaka S (2006) Cilia: tuning in to the cell’s antenna. Curr Biol 16(15):R604–R614
Ou G, Blacque E, Snow JJ, Leroux MR, Scholey JM (2005) Functional coordination of intraflagellar transport motors. Nature 436(7050):583–587
Blacque OE, Leroux MR (2006) Bardet–Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci 63(18):2145–2161
Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LB (1999) Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA 96(9):5043–5048
Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL (2005) Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37(10):1135–1140
Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC (2005) Mkks-null mice have a phenotype resembling Bardet–Biedl syndrome. Hum Mol Genet 14(9):1109–1118
Alton DJ, McDonald P (1973) Urographic findings in the Bardet–Biedl syndrome, formerly the Laurence-Moon-Biedl syndrome. Radiology 109:659–663
Bluett NH, Chantler C, Singer JD, Saxton HM (1977) Congenital renal abnormalities in the Laurence-Moon-Biedl syndrome. Arch Dis Child 52:968–970
O’Dea D (1996) The importance of renal impairment in the natural history of Bardet–Biedl syndrome. Am J Kidney Dis 27(6):776–783
Beales PL, Reid HAS, Griffiths MH, Maher ER, Flinter FA, Woolf AS (2000) Renal cancer and malformations in relatives of patients with Bardet–Biedl syndrome. Nephrol Dial Transplant 15(12):1977–1985
Ersoy A, Kahvecioglu S, Bekar A, Aker S, Akdag I, Dilek K (2005) Primary central nervous system lymphoma in a renal transplant recipient with Bardet–Biedl syndrome. Transplant Proc 37(10):4323–4325
Harnett JD, Green JS, Cramer BC, Johnson G, Chafe L, McManamon P, Farid FR, Pryse-Phillips W, Parfrey PS (1988) The spectrum of renal disease in Laurence-Moon-Biedl syndrome. N Engl J Med 319(10):615–618
Langer RM, Foldes K, Szalay L, Jaray J (2005) Laurence-Moon-Bardet–Biedl syndrome for kidney transplantation at the age of 57 years. Transplant Proc 37(10):4223–4224
Yoder BK, Richards WG, Sweeney WE, Wilkinson JE, Avener ED, Woychik RP (1995) Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc Assoc Am Physicians 107(3):314–323
Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE Jr, Schafer JA, Balkovetz DF (2002) Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282(3):F541–F552
Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G (2004) PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci USA 101(8):2311–2316
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33(2):129–137
Singla V, Reiter JF (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313(5787):629–633
Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37(5):537–543
Hildebrandt F, Omran H (2001) New insights: nephronophthisis-medullary cystic kidney disease. Pediatr Nephrol 16(2):168–176
Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M (2006) Defective planar cell polarity in polycystic kidney disease. Nat Genet 38(1):21–23
Hurley RM, Dery P, Norady MB, Drummond KN (1975) The renal lesion of the Laurence-Moon-Biedl syndrome. J Pediatr 87:206–209
Park TJ, Haigo SL, Wallingford JB (2006) Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and hedgehog signaling. Nat Genet 38(3):303–311
Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B (2006) Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet 38(1):112–117
May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS (2005) Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol 287(2):378–389
Huangfu D, Anderson KV (2005) From the cover: cilia and hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 102(32):11325–11330
Liu A, Wang B, Niswander LA (2005) Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132(13):3103–3111
Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND (2006) GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133(3):569–578
Ou G, Blacque E, Snow JJ, Leroux MR, Scholey JM (2005) Functional coordination of intraflagellar transport motors. Nature 436(7050):583–587
Pan X, Ou G, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM (2006) Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J Cell Biol 174(7):1035–1045
Haraguchi R, Mo R, Hui CC, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G (2001) Unique functions of sonic hedgehog signaling during external genitalia development. Development 128(21):4241–4250
Kang S, Graham JM, Olney AH, Biesecker LG (1997) GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet 15(3):266–268
Vallee RB, Williams JC, Varma D, Barnhart LE (2004) Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol 58(2):189–200
Mak HY, Nelson LS, Basson M, Johnson CD, Ruvkun G (2006) Polygenic control of Caenorhabditis elegans fat storage. Nat Genet 38(3):363–368
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103(14):5466–5471
Albor A, El-Hizawi S, Horn EJ, Laederich M, Frosk P, Wrogemann K, Kulesz-Martin M (2006) The interaction of Piasy with Trim32, an E3-ubiquitin ligase mutated in LGMD2H, promotes Piasy degradation and regulates UVB-induced keratinocyte apoptosis through NFkB. J Biol Chem 281(35):25850–25866
Badano JL, Leitch CC, Ansley SJ, May-Simera H, Lawson S, Lewis RA, Beales PL, Dietz HC, Fisher S, Katsanis N (2006) Dissection of epistasis in oligogenic Bardet–Biedl syndrome. Nature 439(7074):326–330
Acknowledgements
The work of the authors is funded by the Medical Research Council and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tobin, J.L., Beales, P.L. Bardet–Biedl syndrome: beyond the cilium. Pediatr Nephrol 22, 926–936 (2007). https://doi.org/10.1007/s00467-007-0435-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-007-0435-0