Abstract
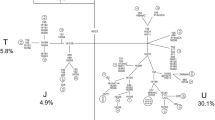
Analyses of mitochondrial DNA (mtDNA) sequences have revealed non-neutral patterns, suggesting that many amino acid mutations in animal mtDNA may be mildly deleterious, but this has not been verified in human clinical series. Since sensorineural hearing impairment (SNHI) is a common manifestation in many of the syndromes caused by mutations in mtDNA, this may be regarded as the phenotype of choice in attempts to detect mutations that may have a mildly deleterious effect on mitochondrial function. We selected 32 subjects from among 117 unrelated SNHI patients with SNHI in maternal relatives by means of family history, determined the entire coding region sequence of mtDNA and compared the sequence variation with that in 32 haplogroup-matched controls taken at random from 192 Finnish sequences. The 32 control sequences differed from the remaining 160 sequences by 36±9 substitutions (mean ± SD), while the difference for the 32 patients was 58±4 substitutions (P=0.005 for difference; Wilcoxon signed rank test). Differences were also found in the number of new haplotypes and new non-synonymous mutations or mutations in tRNA or rRNA genes. A total of 12 rare mtDNA variants were detected in the patients, and only 3 of these were considered to be neutral in effect. It is proposed that increased sequence variation in mtDNA may be a genetic risk factor for SNHI, and the increased frequency of rare haplotypes in these patients points to the presence of mildly deleterious mutations in mtDNA.


Similar content being viewed by others
References
Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N (1999) Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet 23:147
Brown MD, Sun F, Wallace DC (1997) Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 and 14484 mutations on an mtDNA lineage. Am J Hum Genet 30:857–865
Chang-Claude JS, Dong J, Schmidt S, Shayeghi M, Komitowski D, Becher H, Stratton MR, Royer-Pokora B (1998) Using gene carrier probability to select high risk families for identifying germline mutations in breast cancer susceptibility genes. J Med Genet 35:116–121
Elson JL, Andrews RM, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N (2001) Analysis of European mtDNAs for recombination. Am J Hum Genet 68:145–153
Elston RC, Stewart J (1971) A general model for the genetic analysis of pedigree data. Hum Hered 21:523–542
Finnilä S, Hassinen IE, Ala-Kokko L, Majamaa K (2000) Phylogenetic network of the mtDNA haplogroup U in Northern Finland based on sequence analysis of the complete coding region by conformation-sensitive gel electrophoresis. Am J Hum Genet 66:1017–1026
Finnilä S, Lehtonen MS, Majamaa K (2001) Phylogenetic Network for European mtDNA. Am J Hum Genet 68:1475–1484
Fry AJ (1999) Mildly deleterious mutations in avian mitochondrial DNA: evidence from neutrality tests. Evolution 53:1617–1620
Goto Y, Nonaka I, Horai S (1990) A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348:651–653
Herrnstadt C, Elson JL, Fahy E, Preston G, Turnbull DM, Anderson C, Ghosh SS, Olefsky JM, Beal F, Davis RE, Howell N (2002) Reduced-median network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am J Hum Genet 70:1152–1171
Ingman M, Kaessmann H, Pääbo S, Gyllensten U (2000) Mitochondrial genome variation and the origin of modern humans. Nature 408:708–712
Lamminen T, Huoponen K, Sistonen P, Juvonen V, Lahermo P, Aula P, Nikoskelainen E, Savontaus ML (1997) MtDNA haplotype analysis in Finnish families with Leber's hereditary optic neuroretinopathy. Eur J Hum Genet 5:271–279
Lehtonen MS, Uimonen S, Hassinen IE, Majamaa K (2000) Frequency of mitochondrial DNA point mutations among patients with familial sensorineural hearing impairment. Eur J Hum Genet 8:315–318
Lertrit P, Kapsa RMI, Jean-Francois MJB, Thygarajan D, Noer AS, Marzuki S, Byrne E (1994) Mitochondrial DNA polymorphism in disease: a possible contributor to respiratory dysfunction. Hum Mol Genet 3:1973–1981
Majamaa K, Moilanen JS, Uimonen S, Remes AM, Salmela PI, Kärppä M, Majamaa-Voltti KA, Rusanen H, Sorri M, Peuhkurinen KJ, Hassinen IE (1998) Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63:447–454
McDonald JH, Kreitman M (1991) Adaptive protein evolution at the Adh locus in Drosophila. Nature 351:652–654
Nachman MW, Boyer SN, Aquadro CF (1994) Nonneutral evolution at the mitochondrial NADH dehydrogenase subunit 3 gene in mice. Proc Natl Acad Sci U S A 91:6364–6368
Ott J (1991) Analysis of human genetic linkage, revised edn. The Johns Hopkins University Press, Baltimore
Prezant T, Agapian JV, Bohlman MC, Bu X, Öztas S, Qiu W, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, Shohat M, Fischel-Ghodsian N (1993) Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and nonsyndromic deafness. Nat Genet 4:289–294
Pulkes T, Sweeney MG, Hanna MG (2000) Increased risk of stroke in patients with the A12308G polymorphism in mitochondria. Lancet 356:2068–2069
Reid FM, Vernham GA, Jacobs HT (1994) A novel mitochondrial point mutation in a maternal pedigree with sensorineural deafness. Hum Mutat 3:243–247
Schmidt S, Becher H, Chang-Claude J (1998) Breast cancer risk assessment: use of complete pedigree information and the effect of misspecified ages at diagnosis of affected relatives. Hum Genet 102:348–356
Schork NJ, Guo SW (1993) Pedigree models for complex human traits involving the mitochondrial genome. Am J Hum Genet 53:1320–1337
Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC (1990) Myoclonic epilepsy and ragged red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell 61:931–937
Sprinzl M, Hartmann T, Weber J, Blank J, Zeidler R (1989) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 17(suppl):R1–R13
Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S (1998) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 26:148–153
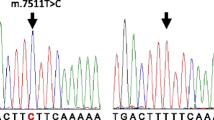
Sue CM, Tanji K, Hadjigeorgiou G, Hadjigeorgiou G, Andreu AL, Nishino I, Krishna S, Bruno C, Hirano M, Shanske S, Bonilla E, Fischel-Ghodsian N, DiMauro S, Friedman R (1999) Maternally inherited hearing loss in a large kindred with a novel T7511C mutation in the mitochondrial DNA tRNASer(UCN) gene. Neurology 52:1905–1908
Thomas AW, Edwards A, Sherratt EJ, Majid A, Gagg J, Alcolado JC (1996) Molecular scanning of candidate mitochondrial tRNA genes in type 2 (non-insulin dependent) diabetes mellitus. J Med Genet 33:253–256
Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, Obinu D, Savontaus ML, Wallace DC (1996) Classification of European mtDNAs from an analysis of three European populations. Genetics 144:1835–1850
Torroni A, Petrozzi M, D'Urbano L, Sellito D, Zeviani M, Carrara F, Carducci C, Leuzzi V, Carelli V, Barboni P, De Negri A, Scozzari R (1997) Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet 60:1107–1121
Tsukihara T, Aoyama H, Yamashita K, Tomizaki T, Yamaguchi H, Shinzawa-Iyoh K, Nakashima R, Yaono R, Yoshikawa S, Yamashita E, Shinzawa-Itoh K (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272:1136–1144
Uimonen S, Moilanen JS,·Sorri M, Hassinen IE, Majamaa K (2001) Hearing impairment in patients with 3243A>G mtDNA mutation: phenotype and rate of progression. Hum Genet 108:284–289
Verhoeven K, Ensink RJH, Tiranti V, Huygen PLM, Johnson DF, Schatteman I, Van Laer L, Verstreken M, Van de Heyning P, Fischel-Ghodsian N, Zeviani M, Cremers CWRJ, Willems PJ, Van Camp G (1999) Hearing impairment and neurological dysfunction associated with a mutation in the mitochondrial tRNASer(UCN) gene. Eur J Hum Genet 7:45–51
Wallace DC (1995) Mitochondrial DNA variation in human evolution, degenerative disease, and aging. Am J Hum Genet 57:201–223
Xia D, Yu C-A, Kim H, Xia J-Z, Kachurin AM, Zhang L, Yu CA, Xian JZ (1997) Crystal structure of the cytochrome bc1 complex from bovine mitochondria. Science 277:60–66
Yamasoba T, Tsukuda K, Oka Y, Kobayashi T, Kaga K (1999) Cochlear histopathology associated with mitochondrial transfer RNALeu(UUR) gene mutation. Neurology 52:1705–1707
Acknowledgements
The expert technical assistance of Ms Irma Vuoti, Ms Kaisu Korhonen and Ms Anja Heikkinen is acknowledged. This work was supported in part by grants from the Medical Research Council of the Academy of Finland, the Sigrid Juselius Foundation, the Emil Aaltonen Foundation and the Finnish Medical Foundation Duodecim.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehtonen, M.S., Moilanen, J.S. & Majamaa, K. Increased variation in mtDNA in patients with familial sensorineural hearing impairment. Hum Genet 113, 220–227 (2003). https://doi.org/10.1007/s00439-003-0966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-003-0966-9