Abstract
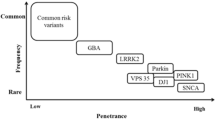
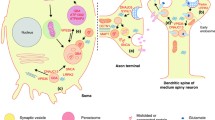
The last 5 years have seen rapid progress in Parkinson’s disease (PD) genetics, with the publication of a series of large-scale genome wide association studies for PD, and evaluation of the roles of the LRRK2 and GBA genes in the aetiology of PD. We are beginning to develop a coherent picture of the interplay of Mendelian and non-Mendelian factors in PD. Pathways involved in mitochondrial quality control (mitophagy), lysosomal function and immune function are emerging as important in the pathogenesis of PD. These pathways represent a target for therapeutic intervention and a way in which the heterogeneity of disease cause, as well as disease mechanism, can be established. In the future, there is likely to be an individualised basis for the treatment of PD, linked to the identification of specific genetic factors.
Similar content being viewed by others
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9(1):13–24
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276(5321):2045–2047 (New York, N.Y.)
Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108
Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I et al (2004) The new mutation, E46 K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J et al (2003) Alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302(5646):841 (New York, N.Y.)
Conway KA (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both alpha -synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci 97(2):571–576
Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon Stoessl A, Farrer MJ (2013) Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord. doi:10.1002/mds.25421
Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, Houlden H, Schapira AH (2013) A novel α-synuclein missense mutation in Parkinson disease. Neurology 80(11):1062–1064. doi:10.1212/WNL.0b013e31828727ba
Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, Proukakis C, Quinn N, Lees AJ, Hardy J, Revesz T, Houlden H, Holton JL (2013) α-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol 125(5):753–769. doi:10.1007/s00401-013-1096-7
Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, Pieri L, Madiona K, Dürr A, Melki R, Verny C, Brice A; for the French Parkinson's Disease Genetics (PDG) Study Group (2013) G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. doi:10.1002/ana.23894
Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH et al (2000) Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol 99(6):663–672
Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S et al (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol 7(7):583–590
Hassin-Baer S, Laitman Y, Azizi E, Molchadski I, Galore-Haskel G, Barak F et al (2009) The leucine rich repeat kinase 2 (LRRK2) G2019S substitution mutation. Association with Parkinson disease, malignant melanoma and prevalence in ethnic groups in Israel. J Neurol 256(3):483–487
Lesage S, Dürr A, Tazir M, Lohmann E, Leutenegger A-L, Janin S et al (2006) LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med 354(4):422–423
Ozelius LJ, Senthil G, Saunders-Pullman R, Ohmann E, Deligtisch A, Tagliati M et al (2006) LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N Engl J Med 354(4):424–425
Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simón J, Van der Brug M et al (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44(4):595–600
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S et al (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44(4):601–607
Latourelle JC, Sun M, Lew MF, Suchowersky O, Klein C, Golbe LI et al (2008) The Gly2019Ser mutation in LRRK2 is not fully penetrant in familial Parkinson’s disease: the GenePD study. BMC Med 6:32
Cookson MR (2010) The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci 11(12):791–797. doi:10.1038/nrn2935
Lewis PA, Manzoni C (2012) LRRK2 and human disease: a complicated question or a question of complexes? Sci Signal 5(207):pe2. doi:10.1126/scisignal.2002680
Lewis PA (2009) The function of ROCO proteins in health and disease. Biol Cell/Under Auspices Eur Cell Biol Organ 101(3):183–191
Plun-Favreau H, Lewis PA, Hardy J, Martins LM, Wood NW (2010) Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet 6(12):e1001257. doi:10.1371/journal.pgen.1001257
Bajaj A, Driver JA, Schernhammer ES (2010) Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control: CCC 21(5):697–707
Morris LGT, Veeriah S, Chan TA (2010) Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene 29(24):3453–3464
Saunders-Pullman R, Barrett MJ, Stanley KM, Luciano MS, Shanker V, Severt L et al (2010) LRRK2 G2019S mutations are associated with an increased cancer risk in Parkinson disease. Mov Disord: Off J Mov Disord Soc 25(15):2536–2541
Vilariño-Güell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ et al (2011) VPS35 mutations in Parkinson disease. Am J Hum Genet 89(1):162–167
Lesage S, Condroyer C, Klebe S, Honoré A, Tison F, Brefel-Courbon C, Dürr A, Brice A; French Parkinson’s Disease Genetics Study Group (2012) Identification of VPS35 mutations replicated in French families with Parkinson disease. Neurology 78(18):1449–1450. doi:10.1212/WNL.0b013e318253d5f2
Sharma M, Ioannidis JP a, Aasly JO, Annesi G, Brice A, Bertram L et al (2012) A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J Med Genet 49(11):721–726
Sheerin U-M, Charlesworth G, Bras J, Guerreiro R, Bhatia K, Foltynie T et al (2012) Screening for VPS35 mutations in Parkinson’s disease. Neurobiol Aging 33(4):838.e1–838.e5
Ando M, Funayama M, Li Y, Kashihara K, Murakami Y, Ishizu N et al (2012) VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov Disord: Off J Mov Disord Soc 27(11):1413–1417
Chartier-Harlin M-C, Dachsel JC, Vilariño-Güell C, Lincoln SJ, Leprêtre F, Hulihan MM et al (2011) Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet 89(3):398–406
Tucci A, Charlesworth G, Sheerin U-M, Plagnol V, Wood NW, Hardy J (2012) Study of the genetic variability in a Parkinson’s disease gene: EIF4G1. Neurosci Lett 518(1):19–22
Nuytemans K, Bademci G, Inchausti V, Dressen A, Kinnamon DD, Mehta A et al (2013) Whole exome sequencing of rare variants in EIF4G1 and VPS35 in Parkinson disease. Neurology 80(11):982–989
Lesage S, Condroyer C, Klebe S, Lohmann E, Durif F, Damier P et al (2012) EIF4G1 in familial Parkinson’s disease: pathogenic mutations or rare benign variants? Neurobiol Aging 33(9):2233.e1–2233.e5
Schulte EC, Mollenhauer B, Zimprich A, Bereznai B, Lichtner P, Haubenberger D et al (2012) Variants in eukaryotic translation initiation factor 4G1 in sporadic Parkinson’s disease. Neurogenetics 13(3):281–285
Kitada T, Askawa S, Hattori N, Matsumine H, Yokochi M, Mizuno Y et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile Parkinsonism. Nature 392:605–608
Kilarski LL, Pearson JP, Newsway V, Majounie E, Knipe MDW, Misbahuddin A et al (2012) Systematic Review and UK-Based Study of PARK2 (parkin), PINK1, PARK7 (DJ-1) and LRRK2 in early-onset Parkinson’s disease. Mov Disord 27(12):1522–1529
Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C (2010) Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat 31(7):763–780
Shimura H, Hattori N, Kubo S i, Mizuno Y, Asakawa S, Minoshima S et al (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25(3):302–305
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S et al (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304(5674):1158–1160 (New York, N.Y.)
Schon E a, Przedborski S (2011) Mitochondria: the next (neurode) generation. Neuron 70(6):1033–1053
Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H et al (2006) Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet 15(6):883–895
Shin J-H, Ko HS, Kang H, Lee Y, Lee Y-I, Pletinkova O et al (2011) PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell 144(5):689–702
Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH et al (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441(7097):1162–1166
Park J, Lee SB, Lee S, Kim Y, Song S, Kim S et al (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441(7097):1157–1161
Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K et al (2009) PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33(5):627–638
Bonifati V, Rizzu P, Van Baren MJ, Schaap O, Breedveld GJ, Krieger E et al (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299(5604):256–259 (New York, N.Y.)
Canet-Avilés RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S et al (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 101(24):9103–9108
Miyakawa S, Ogino M, Funabe S, Uchino A, Shimo Y, Hattori N et al (2013) Lewy body pathology in a patient with a homozygous Parkin deletion. Mov Disord: Off J Mov Disord Soc 28(3):388–391
Doherty KM, Silveira-Moriyama L, Parkkinen L, Healy DG, Farrell M, Mencacci NE et al (2013) Parkin disease: A clinicopathologic entity? JAMA Neurol 4:1–9
Ahlskog JE (2009) Parkin and PINK1 parkinsonism may represent nigral mitochondrial cytopathies distinct from Lewy body Parkinson’s disease. Parkinsonism Relat Disord 15(10):721–727
Ramirez A, Heimbach A, Gründemann J, Stiller B, Hampshire D, Cid LP et al (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 38(10):1184–1191
Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D (2012) Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci: Off J Soc Neurosci 32(12):4240–4246
Paisán-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E et al (2010) Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord: Off J Mov Disord Soc 25(12):1791–1800
Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW et al (2009) Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol 65(1):19–23
Kauther KM, Höft C, Rissling I, Oertel WH, Möller JC (2011) The PLA2G6 gene in early-onset Parkinson’s disease. Mov Disord: Off J Mov Disord Soc 26(13):2415–2417
Di Fonzo A, Dekker MCJ, Montagna P, Baruzzi A, Yonova EH (2009) Correia Guedes L, et al. FBXO7 mutations cause autosomal recessive, early-onset parkinsonian-pyramidal syndrome. Neurology 72(3):240–245
Deng H, Liang H, Jankovic J (2012) F-Box Only Protein 7 Gene in Parkinsonian-Pyramidal Disease. Archives Neurol 1:1–5
Simón-Sánchez J, Kilarski LL, Nalls MA, Martinez M, Schulte C, Holmans P et al (2012) Cooperative genome-wide analysis shows increased homozygosity in early onset Parkinson’s disease. PLoS ONE 7(3):e28787
Simón-Sánchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D et al (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41(12):1308–1312
Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M et al (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 41(12):1303–1307
Pankratz N, Beecham GW, DeStefano AL, Dawson TM, Doheny KF, Factor SA et al (2012) Meta-analysis of Parkinson’s disease: identification of a novel locus, RIT2. Ann Neurol 71(3):370–384
Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW et al (2009) Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet 124(6):593–605
Edwards TL, Scott WK, Almonte C, Burt A, Powell EH, Beecham GW et al (2010) Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann Hum Genet 74(2):97–109
Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D et al (2010) Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet 42(9):781–785
Saad M, Lesage S, Saint-Pierre A, Corvol J-C, Zelenika D, Lambert J-C et al (2011) Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson’s disease in the European population. Hum Mol Genet 20(3):615–627
Spencer CCA, Plagnol V, Strange A, Gardner M, Paisan-Ruiz C, Band G et al (2011) Dissection of the genetics of Parkinson’s disease identifies an additional association 5’ of SNCA and multiple associated haplotypes at 17q21. Hum Mol Genet 20(2):345–353
Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin U-M, Saad M et al (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377(9766):641–649
Plagnol V, Nalls MA, Bras JM, Hernandez DG, Sharma M, Sheerin U-M et al (2011) A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS Genet 7(6):e1002142
Do CB, Tung JY, Dorfman E, Kiefer AK, Drabant EM, Francke U et al (2011) Web-based genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet 7(6):e1002141
Liu X, Cheng R, Verbitsky M, Kisselev S, Browne A, Mejia-Sanatana H et al (2011) Genome-wide association study identifies candidate genes for Parkinson’s disease in an Ashkenazi Jewish population. BMC Med Genet 12:104
Pihlstrøm L, Axelsson G, Bjørnarå KA, Dizdar N, Fardell C, Forsgren L et al (2012) Supportive evidence for 11 loci from genome-wide association studies in Parkinson’s disease. Neurobiol Aging 34(6):1708.e7–1708.e13
Sharma M, Ioannidis JPA, Aasly JO, Annesi G, Brice A, Van Broeckhoven C et al (2012) Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology 79(7):659–667
Hardy J, Singleton A (2009) Genomewide association studies and human disease. N Engl J Med 360(17):1759–1768
Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH et al (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447(7146):799–816
Keller MF, Saad M, Bras J, Bettella F, Nicolaou N, Simón-Sánchez J et al (2012) Using genome-wide complex trait analysis to quantify “missing heritability” in Parkinson’s disease. Hum Mol Genet 21(22):4996–5009
Evangelou E, Maraganore DM, Annesi G, Brighina L, Brice A, Elbaz A et al (2010) Non-replication of association for six polymorphisms from meta-analysis of genome-wide association studies of Parkinson’s disease: large-scale collaborative study. Am J Med Genet Part b, Neuropsychiatr Genet: Off Publ Int Soc Psychiatr Genet 153B(1):220–228
Tayebi N, Walker J, Stubblefield B, Orvisky E, LaMarca ME, Wong K et al (2003) Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab 79(2):104–109
Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E et al (1996) Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM: Mon J Assoc Physicians 89(9):691–694
Tayebi N, Callahan M, Madike V, Stubblefield BK, Orvisky E, Krasnewich D et al (2001) Gaucher disease and Parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab 73(4):313–321
Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH et al (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain: J Neurol 132(Pt 7):1783–1794
Sidransky E, Nalls M a, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER et al (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361(17):1651–1661
Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T et al (2013) Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain: J Neurol 136(Pt 2):392–399
McNeill A, Duran R, Hughes DA, Mehta A, Schapira AHV (2012) A clinical and family history study of Parkinson’s disease in heterozygous glucocerebrosidase mutation carriers. J Neurol Neurosurg Psychiatr 83(8):853–854
Gegg ME, Burke D, Heales SJR, Cooper JM, Hardy J, Wood NW et al (2012) Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol 72(3):455–463
Singleton A, Hardy J (2011) A generalizable hypothesis for the genetic architecture of disease: pleomorphic risk loci. Hum Mol Genet 20(2):158–162
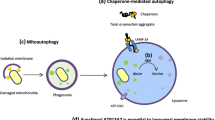
Deas E, Wood NW, Plun-Favreau H (2010) Mitophagy and Parkinson’s disease: the PINK1—parkin link. Biochim Biophys Acta 1813(4):623–633. doi:10.1016/j.bbamcr.2010.08.007
Whitworth AJ, Pallanck LJ (2009) The PINK1/Parkin pathway: a mitochondrial quality control system? J Bioenergetics Biomembranes 41(6):499–503
Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SHY et al (2011) PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet 20(5):867–879
Sekine S, Kanamaru Y, Koike M, Nishihara A, Okada M, Kinoshita H et al (2012) Rhomboid protease PARL mediates the mitochondrial membrane potential loss-induced cleavage of PGAM5. J Biol Chem 287(41):34635–34645
Narendra D, Tanaka A, Suen D-F, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183(5):795–803
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14
Gegg ME, Cooper JM, Chau K-Y, Rojo M, Schapira AHV, Taanman J-W (2010) Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19(24):4861–4870
Ziviani E, Tao RN, Whitworth AJ (2010) Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA 107(11):5018–5023
Narendra D, Walker JE, Youle R (2012) Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol 4(11). doi:10.1101/cshperspect.a011338
Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P et al (2012) Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet 8(3):e1002537
Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D et al (2011) PINK1 and parkin target miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147(4):893–906
Sun Y, Vashisht AA, Tchieu J, Wohlschlegel JA, Dreier L (2012) Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J Biol Chem 287(48):40652–40660
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ et al (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12(2):119–131
Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146(1):37–52. doi:10.1016/j.cell.2011.06.001
Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F et al (2013) A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum Mol Genet 22(5):1039–1049
Saiki M, Baker A, Williams-Gray CH, Foltynie T, Goodman RS, Taylor CJ et al (2010) Association of the human leucocyte antigen region with susceptibility to Parkinson’s disease. J Neurol Neurosurg Psychiatr 81(8):890–891
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Mov Disord: Off J Mov Disord Soc 23(4):474–483
Charlesworth G, Gandhi S, Bras JM, Barker RA, Burn DJ, Chinnery PF et al (2012) Tau acts as an independent genetic risk factor in pathologically proven PD. Neurobiol Aging 33(4):838.e7–838.e11
Acknowledgments
Our work is funded by Parkinson’s UK (Grants 8047 and J-1101) and the Medical Research Council UK (G0700943, G1100643).
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lubbe, S., Morris, H.R. Recent advances in Parkinson’s disease genetics. J Neurol 261, 259–266 (2014). https://doi.org/10.1007/s00415-013-7003-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7003-2