Abstract
We assessed the value of three-dimensional (3D) dynamic magnetic resonance angiography (MRA) for the follow-up of patients with radiosurgically treated cerebral arteriovenous malformations (AVMs). Fifty-four patients with cerebral AVMs treated by radiosurgery (RS) were monitored using conventional catheter angiography (CCA) and 3D dynamic MRA with sensitivity encoding based on the parallel imaging. Cerebral AVM was qualitatively classified by two radiologists into one of five categories in terms of residual nidus size and persistence of early draining vein (I, >6 cm; II, 3–6 cm; III, <3 cm; IV, isolated early draining vein; V, complete obliteration). 3D MRA findings showed a good agreement with CCA in 40 cases (κ=0.62). Of 23 nidus detected on CCA, 3D dynamic MRA showed 14 residual nidus. Of 28 occluded nidus on 3D dynamic MRA, 22 nidus were occluded on CCA. The sensitivity and specificity of 3D dynamic MRA for the detection of residual AVM were 81% and 100%. 3D dynamic MRA after RS may therefore be useful in association with MRI and can be repeated as long as opacification of the nidus or early venous drainage persists, one CCA remaining indispensable to affirm the complete occlusion at the end of follow-up.
Similar content being viewed by others
Introduction
Complete obliteration of cerebral arteriovenous malformations (AVMs) after radiosurgery (RS) can occur as early as 4 months or as late as 5 years after treatment; this vasoocclusive effect often developing slowly and progressively. Because the risk of bleeding persists as long as complete obliteration is not obtained, the time course of nidus changes after RS is crucial for patient management. Conventional catheter angiography (CCA) is essential at the end of follow-up to confirm complete occlusion and to stop annual imaging follow-up [1]. For intermediate controls magnetic resonance angiography (MRA) with time of flight (TOF) or phase contrast techniques or computed tomography angiography (CTA) are usually used as an alternative to CCA for the assessment of cerebral vascular diseases [2–5]. For the assessment of cerebral AVMs, however, most teams consider this technique as an additional tool since its limited temporal resolution and lack of hemodynamic information do not allow the arterial phase to be separated from the venous phase of intracranial circulation in comparison with CCA [6, 7]. Thus the reduction in size or disappearance of the nidus and/or the persistence of early venous drainage, which are essential criteria to affirm recovery, are difficult to determine with precision with MRA.
Magnetic resonance digital subtraction angiography (DSA) and time-resolved contrast-enhanced MRA sequences have recently been developed to circumvent this drawback and are used in vascular diseases such as AVM, dural fistulas and venous thrombosis [8–13]. They give hemodynamic information and can play an important role in evaluating the decrease in nidus size and/or the disappearance of venous drainage. This method has several limitations, however, including the poor visualization of small vessels or shunts because of partial-volume effects, the two-dimensional acquisition with a large slice thickness and the limited spatial resolution. Recent developments using three-dimensional (3D) dynamic MRA acquisition with multiple surface coils and parallel imaging methods such as sensitivity encoding help to decrease imaging time and maintain spatial resolution [14–18].
The purpose of this study was to evaluate the reliability of 3D dynamic MRA using sensitivity encoding for the assessment of cerebral arteriovenous malformations after RS.
Materials and methods
Population
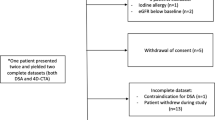
Between October 2003 and May 2004, 54 consecutive patients (28 men, 26 women; median age 40 years, range 21–72) with cerebral AVMs treated by RS were assessed by CCA and 3D dynamic MRA. The period between the two examinations was less than 48 h. Presenting symptoms were hemorrhage in 15, seizures and headache in 6 each, and vertigo in one; 26 were asymptomatic. Twelve patients had previously undergone endovascular embolization, and one had had stereotactic RS. The nidus was located in the supratentorial region in 46 cases (17 temporal lobe, 12 frontal lobe, 7 occipital lobe, 3 parietal lobe, 4 corpus callosum, 2 basal nucleus, 1 lateral ventricles) and in the infratentorial region in 8. Follow-up CCA or 3D dynamic MRA was performed in patients at an mean of 4.3 years (median 3 years, range 1–14) after stereotactic RS. Full approval was received from the local ethics committee review board and written informed consent was obtained from all patients.
Radiosurgery
The RS technique was similar in all cases. RS was performed with a GE-CGR Saturne 43 linear accelerator (Linac) system with eight additional 6- to 20-mm collimators. Patients were irradiated in the Betti [19] armchair with the head positioned by the Talairach stereotactic frame. A dose of 25 Gy was delivered at the periphery of the nidus, delineated on the pretherapeutic angiogram. This dose corresponds to 60–70% of the peripheral isodose range. The maximal dose given ranged from 14 to 25 Gy (mean 21.9). The number of isocenters ranged from 1 to 4 (mean 1.6) and the mean maximal length of cerebral AVMs on pretherapeutic angiograms from 5 to 40 mm (mean 19 mm).
MR protocol
MRI was performed on a 1.5-T superconducting system (Signa Excite Echospeed+, GE Medical Systems, Waukesha, Wis., USA). Routine MRI included pre- and postcontrast T1-weighted (performed after 3D dynamic MRA), spin-echo and T2-weighted fast spin-echo pulse sequences. 3D dynamic MRA with array spatial sensitivity encoding techniques (ASSET) was acquired in the sagittal and coronal plane. A volume of 20 dynamic images, without previous calculation of circulation time of contrast media, was obtained to track the contrast bolus and was repeated 18 times every 1.7 s. Imaging parameters for the dynamic images were 3.6 1.4 25° (TR/TE/flip), a 300×300 mm field of view sufficient to image the whole brain, a 320×192 acquisition matrix, a slice section thickness of 10 mm with 20 overlapped sections resulting in a 10-cm-thick volume covering the whole brain to visualize both the deep and the superficial drainage. Spatial resolution was 0.9375×1.562×10 mm3 with 0.58×0.58×5 mm3 after zero filling interpolation. 3D dynamic MRA was initiated 5 s after the start of 10 ml gadolinium (Dotarem, Guerbet, France) administered intravenously as a bolus at a rate of 3 ml/s using a Medrad Spectris power injector (Medrad, Indianola, Pa., USA) followed by 10 ml saline flush. A separate contrast bolus was used for each anatomical plane. After the MRA sequences had been obtained the data were transferred to a workstation (Advantage Windows 4.1; GE Medical Systems). Image processing included subtraction of the first volume from the series and reconstructions of the maximum intensity projection (MIP) obtained and consisted of lateral and anteroposterior projections.
Conventional catheter angiography
CCA was performed with a 4-F catheter via a femoral arterial approach (BV 3000, Philips Medical Systems), with a filming rate of two or three images per second. Follow-up angiography included a selective injection of internal and common carotid or vertebral arteries in anteroposterior and lateral views completed by additional views where necessary. Images were printed with a 512×512 matrix and an field of view of 17 cm. For each projection an 8- to 16-ml bolus of iodinated contrast material (Xenetix, Guerbet, France) was injected at a rate of between 3 and 6 ml/s using a power injector.
Image evaluation
3D dynamic MR angiograms were analyzed independently by two radiologists (O.N., J.Y.G.) blinded to the CCA images. One examiner (D.T.) who was blinded to the MRA results examined the CCA which was used as reference. 3D dynamic MRA image quality was graded according to the effectiveness of arterial venous separation of normal vessels: high when the separation was optimal; in this case each of two structures (arteries, veins) was clearly localized and individualized on MR angiograms, low when two structures (arteries, veins) were difficult to separate.
Cerebral AVMs were analyzed in three projections (craniocaudal, anteroposterior and lateral view) but only the largest dimension of the nidus was provided according to the Spetzler and Martin [20] classification and then classified into one of three groups: small (<3 cm), medium (3–6 cm) and large (>6 cm). Moreover, an isolated early venous drainage without nidus was noted. Each cerebral AVM was finally assigned to one of the following five categories: I, when nidus size was more than 6 cm; II, when nidus size was between 3 and 6 cm; III, when nidus size was less than 3 cm; IV, when only an isolated early draining vein was detected; and V when a complete obliteration of the nidus was observed (100% reduction).
Statistical analysis
All statistical studies were performed using MedCalc software (MedCalc, Mariakerke, Belgium). The first step of the analysis consisted of an evaluation of the level of interobserver agreement for each set of 3D dynamic MRA by means of the κ statistic. The second step consisted of a comparison between 3D dynamic MRA and CCA in five categories using the same statistical tests κ higher than 0.6 suggested a substantial agreement, and values higher than 0.8 indicated an excellent agreement; P values lower than 0.05 were regarded as significant. The third step consisted in determining the sensitivity and specificity of 3D dynamic MRA for the detection of a residual cerebral AVM (we considered two classes for the evaluation: category V and categories I-IV).
Results
Image quality
Successful 3D dynamic MRAs were obtained in all patients. No degradation of image quality was noted after injection of the contrast for the second run. The overall image quality of 3D dynamic MRA images was judged to be high in 51 patients and low in 3 patients but in the latter cases did not prevent image interpretation.
Interobserver agreement
Interobserver agreement was good (κ=0.69, 95% confidence interval 0.515–0.863). Discrepancies between the two examiners were noted in ten cases (one observer overestimated the nidus size in four cases and the other overestimated it in another six). In these cases an additional reading was performed by both examiners to reach a consensus.
Intertechnique agreement
Among the 54 cerebral AVMs CCA showed a complete obliteration of the nidus in 22 (group V), residual nidus in 23 (four in group II, 19 in group III), and isolated early draining vein in 9 (group IV; Tables 1, 2). On 3D dynamic MR angiography the nidus was shown to be completely obliterated in 28 patients (group V); a residual nidus was observed in 14 (4 in group II, 10 in group III), and an early draining vein persisted in 12 (group IV). 3D dynamic MRA findings showed a good agreement with CCA in 40/54 cases (κ=0.62, 95% confidence interval 0.45–0.79; Figs. 1, 2, 3). In eight cases 3D dynamic MRA resulted in an overestimation of the size reduction in the nidus and detected only a venous drainage (Fig. 4), and in six cases it resulted in cerebral AVMs being misclassified in the complete occlusion group whereas CCA showed a persistent nidus or venous drainage (Fig. 5).
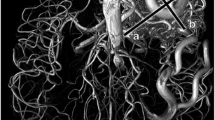
A 46-year-old man with a left frontal cerebral AVM. Post-RS study at 5 years. a Conventional angiogram with lateral view showing a small-sized nidus with one feeding artery deriving from the middle cerebral artery and multiple superficial veins draining into the superior sagittal sinus (arrows) and left lateral sinus (arrowhead). b 3D dynamic MRA images with sagittal MIP reconstructions showing feeding arteries and multiple superficial draining veins in agreement with CCA findings (arrows and had arrows)
A 32-year old woman with a left temporal cerebral AVM. Post-RS study at 3 years. a CCA with lateral view showing a small-sized nidus with feedings deriving from the middle cerebral artery and with three superficial veins draining, two into the superior sagittal sinus (arrows) and one into the left lateral sinus (headarrow). b 3D dynamic MRA images with sagittal MIP reconstructions showing feeding arteries and three superficial draining veins in agreement with CCA findings (arrows, arrowheads)
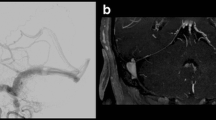
A 29-year-old man with a cerebral AVM. RS study at 2 years. a, b Conventional angiograms with lateral (a) and anteroposterior (b) views showing a small nidus fed by the posterior cerebral artery via the posterior communicating artery and drained by a single vein. c, d 3D dynamic MRA images in sagittal (c) and coronal (d) MIP reconstructions do not show the nidus but show a persisting single draining vein (arrow)
A 44-year-old woman with a cerebral AVM. RS study at 3 years. a Conventional angiogram with lateral pre-RS views showing a cerebral AVM fed by the posterior cerebral artery. b At 12 months after RS conventional angiogram with lateral view shows only a single isolated draining vein. c 3D dynamic MRA images during the passage of the contrast bolus with sagittal MIP reconstructions do not demonstrate the draining vein
The sensitivity and specificity of 3D dynamic MRA for the detection of a residual nidus or venous drainage were 81% (95% confidence interval 63–92%) and 100% (95% confidence interval 82–100%), respectively.
Discussion
Our study showed 3D dynamic MRA to be highly specific for the detection of cerebral AVM with persistent nidus or venous drainage after RS. All circulating cerebral AVMs detected on 3D dynamic MRA were confirmed by CCA. RS produces cerebral AVM obliteration by inducing a disease process in the nidus, leading to gradual thickening of the vessels until occlusion occurs. The postradiosurgical response in terms of nidus size appears to be highly variable. Cerebral AVM changes are delayed after the time of treatment with a slowly and progressive shrinking of the nidus. The vasoocclusive effects usually occur between 1 and 3 years after RS depending on the initial size of the nidus [21]. Incomplete obliteration remains a problem because residual arteriovenous malformations present a similar or even greater risk of hemorrhage [22]. Complete obliteration of cerebral AVM must be documented, and this may need to be repeated several times in the follow-up after RS because of the progressive effect of irradiation.
In routine evaluation no invasive techniques such as MRA or CTA are used. 3D TOF MRA permits delineation of the vascular architecture and in conjunction with CCA is used in planning stereotactic RS and radiation dose [1, 23]. One advantage of this technique is to provide 3D information on the nidus. However, TOF sequences are less detective in detecting small vessels and the slow flows [24, 25]. An alternative approach is contrast enhanced MRA, and some authors suggest an additional injection with TOF MRA [26–28]. Those contrasts agents are so used to selectively shorten the T1 of the blood below the T1 value of the stationary tissues. Furthermore, T1-shortening effect of gadolinium helps to overcome the saturation effects of slow flow. Another technique is contrast-enhanced 3D MRA, based on a single acquisition of the imaging volume during the maximum concentration of gadolinium within the arteries. It provides high contrast images with minimized flow-related artifacts.
CTA has proven effective because it provides the simultaneous visualization of arterial and venous anatomy depicting an entire arteriovenous malformation including the nidus with its arterial feeding vessels and venous drainage supply [5, 29–33]. Three-dimensional reconstructions and multiplanar vascular MIPs display the complex vasculature and show the relationship of the arteriovenous malformation nidus with eloquent cortex. CTA is a useful diagnostic technique, both during stereotactic localization before surgical resection or radiosurgical treatment and during the imaging follow-up after RS. However, as with MRA, this technique does not allow a hemodynamic assessment. Moreover, implementation of Euratom Directive 97/43 requires increased scrutiny of the use CT imaging given its relatively high radiation exposure, and it is recommended that techniques such as MRA that do not involve the use of radiation should be used whenever possible [34, 35].
However, a major drawback of MRA and CTA is the absence of hemodynamic information at the different phases of intracranial circulation during the passage of the contrast agent. This point is crucial for the evaluation of cerebral AVMs, particularly for the follow-up after RS [36, 37]. To circumvent this, time-resolved contrast-enhanced MRA was developed. Preliminary reports suggested that this method could be a useful tool for the follow-up of patients with cerebral AVMs [9, 15, 18, 38–40]. The technique provides simultaneous opacification of vertebral and carotid arteries, allowing the nidus to be accurately delineated. It is particularly useful for the evaluation of posterior cerebral AVMs, which requires selective catheterization of both vertebral and carotid arteries by the use of CCA. Hemorrhage or adverse radiation effects, which may cause an underestimation of cerebral AVM obliteration on standard MRA, do not prevent image interpretation on dynamic MR images [41] because subtraction of the first volume erases the T1 effect of methemoglobin and shows only the nidus. No studies have compared CCA and dynamic MRA in a large population treated exclusively by RS. However, despite the potential interest of time-resolved contrast-enhanced MRA, the low spatial resolution inherent in the 2D technique results in a poor visualization of small vessels or shunts.
Recent technical developments including parallel imaging such as SENSE (sensitivity encoding) [14], ASSET [42], and simultaneous acquisition of spatial harmonics (SMASH) [16, 43] combined with 3D ultrafast sequences have dramatically improved both temporal and spatial resolution of dynamic MRA. These technique use multiple receiver coils to exploit information related to the distinct spatial sensitivity of each element of the coil and fast parallel-encoding imaging methods. The speed improvement depends on a factor equal to the number of parallel receiver coils used. This technique has numerous advantages. A temporal resolution of 1.7 s may be achieved using an ASSET factor set to 4. This allows hemodynamic information similar to that obtained by CCA with separation of arterial and venous phases of angiograms thereby facilitating the understanding of intracranial hemodynamic changes [44]. Spatial resolution can also be optimized with submillimeter in-plane resolution and slice thickness of 5 mm leading to a voxel size of 0.58×0.58×5 mm3 after interpolation. Other parameters include an imaging coverage of 10 cm suitable for evaluating the whole cerebral AVM without the need for view sharing or temporal interpolation. A small amount of gadolinium (10 ml) is injected at the start of each projection, leading to optimal image quality. At a fixed injection rate and with similar dynamic acquisition time parallel imaging techniques require less contrast agent to maintain appropriate vessel opacification [45]. The duration of plateau concentration of contrast agent is maintained at least over the duration of multiple dynamic acquisitions used in view sharing and interpolation [46]. The last advantage is that morphological MR images are acquired on the same time and can sensitively depict subtle changes of the brain parenchyma in the vicinity of these radiosurgically treated AVMs [47, 48].
The follow-up imaging of cerebral AVMs is problematic because of the difficulty in performing an annual examination so long as a nidus or early venous drainage persists, especially when an invasive technique is involved. The disparity of the results in different studies can be explained by the incomplete imaging follow-up of patients after radiosurgery [49, 50]. Patients have the impression of being cured and as a result may be lost to follow-up. The imaging follow-up might be seen as more acceptable if it consisted of a single 3D dynamic MRA. Moreover, the cumulative irradiation of repeated CCA (diagnosis stage, interventional procedures and follow-up imaging stage) can be reduced by using noninvasive techniques such as 3D dynamic MRA between the initial diagnostic CCA and the final CCA confirming the complete disappearance of the cerebral AVM.
Compared to CCA, 3D dynamic MRA has several limitations. First, the time resolution is limited to one frame per 1.7 s compared to one frame per 0.5 s in the case of CCA. This major drawback may explain the difficulty in isolating venous drainage, distinguishing it from normal venous drainage and delimiting nidus of less than 1 cm and slow flow or residual small fistulae. This limitation is particularly pronounced in the cases of very small AVMs, because the only finding can be often an early venous drainage. In AVMs with a precocious venous drainage the contrast bolus may be missed with a temporal resolution of 1.7 s per frame.
Another limitation of the technique concerns the spatial resolution. 3D dynamic MRA cannot detect intranidal aneurysms, which are often present and lead to a higher risk of hemorrhage. Nevertheless, the impact of partial or complete cerebral AVM obliteration on the natural history of such aneurysms remains unclear and it is difficult to differentiate a late-filling aneurysm from an early-filling venous pouch. CCA is therefore recommended at the initial diagnosis stage to show aneurysm.
In conclusion, our study shows that 3D dynamic MRA with the parallel imaging technique in combination with standard MRI sequences is a valuable method and is suitable for the evaluation of radiosurgically treated cerebral AVMs as long as an opacification of the nidus or early venous drainage persists. When 3D dynamic MRA findings appear to indicate complete obliteration CCA must be performed for confirmatory purposes.
References
Byrne JV (2005) Cerebrovascular malformations. Eur Radiol 15:448–452
Korosec FR, Turski PA, Carroll TJ, Mistretta CA, Grist TM (1999) Contrast-enhanced MR angiography of the carotid bifurcation. J Magn Reson Imaging 10:317–325
Remonda L, Heid O, Schroth G (1998) Carotid artery stenosis, occlusion, and pseudo-occlusion: first-pass, gadolinium-enhanced, three-dimensional MR angiography-preliminary study. Radiology 209:95–102
Remonda L, Senn P, Barth A, Arnold M, Lovblad KO, Schroth G (2002) Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. Am J Neuroradiol 23:213–219
Sanelli PC, Mifsud MJ, Stieg PE (2004) Role of CT angiography in guiding management decisions of newly diagnosed and residual arteriovenous malformations. Am J Roentgenol 183:1123–1126
Duran M, Schoenberg SO, Yuh WT, Knopp MV, van Kaick G, Essig M (2002) Cerebral arteriovenous malformations: morphologic evaluation by ultrashort 3D gadolinium-enhanced MR angiography. Eur Radiol 12:2957–2964
Farb RI, McGregor C, Kim JK, Laliberte M, Derbyshire JA, Willinsky RA, Cooper PW, Westman DG, Cheung G, Schwartz ML, Stainsby JA, Wright GA (2001) Intracranial arteriovenous malformations: real-time auto-triggered elliptic centric-ordered 3D gadolinium-enhanced MR angiography-initial assessment. Radiology 220:244–251
Griffiths PD, Hoggard N, Warren DJ, Wilkinson ID, Anderson B, Romanowski CA (2000) Brain arteriovenous malformations: assessment with dynamic MR digital subtraction angiography. Am J Neuroradiol 1892–1899
Klisch J, Strecker R, Hennig J, Schumacher M (2000) Time-resolved projection MRA: clinical application in intracranial vascular malformations. NeuroRadiology 42:104–107
Aoki S, Yoshikawa T, Hori M, Ishigame K, Nambu A, Kumagai H, Araki T (2000) Two-dimensional thick-slice MR digital subtraction angiography for assessment of cerebrovascular occlusive diseases. Eur Radiol 10:1858–1864
Tsuchiya K, Katase S, Yoshino A, Hachiya J (2000) MR digital subtraction angiography of cerebral arteriovenous malformations. Am J Neuroradiol 21:707–711
Mori H, Aoki S, Okubo T, Hayashi N, Masumoto T, Yoshikawa T, Tago M, Shin M, Kurita H, Abe O, Ohtomo K (2003) Two-dimensional thick-slice MR digital subtraction angiography in the assessment of small to medium-size intracranial arteriovenous malformations. NeuroRadiology 45:27–33
Warren DJ, Hoggard N, Walton L, Radatz MW, Kemeny AA, Forster DM, Wilkinson ID, Griffiths PD (2001) Cerebral arteriovenous malformations: comparison of novel magnetic resonance angiographic techniques and conventional catheter angiography. Neurosurgery 48:973–982
Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P (1999) SENSE: sensitivity encoding for fast MRI. Magn Reson Med 42:952–962
Weiger M, Pruessmann KP, Kassner A, Roditi G, Lawton T, Reid A, Boesiger P (2000) Contrast-enhanced 3D MRA using SENSE. J Magn Reson Imaging 12:671–677
Sodickson DK, Manning WJ (1997) Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med 38:591–603
Chen Q, Quijano CV, Mai VM, Krishnamoorthy SK, Li W, Storey P, Edelman RR (2004) On improving temporal and spatial resolution of 3D contrast-enhanced body MR angiography with parallel imaging. Radiology 231:893–899
Tsuchiya K, Aoki C, Fujikawa A, Hachiya J (2004) Three-dimensional MR digital subtraction angiography using parallel imaging and keyhole data sampling in cerebrovascular diseases: initial experience. Eur Radiol 14:1494–1497
Betti OO (1987) Treatment of arteriovenous malformations with the linear accelerator. Appl Neurophysiol 50:262
Schlienger M, Lefkopoulos D, Nataf F, Mammar H, Missir O, Meder JF, Huart J, Platoni P, Deniaud-Alexandre E, Merienne L (2003) Repeat linear accelerator radiosurgery for cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys 56:529–536
Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD (2002) An analysis of the dose-response for arteriovenous malformation radiosurgery and other factors affecting obliteration. Radiother Oncol 63:347–354
Anzalone N, Scomazzoni F, Strada L, Patay Z, Scotti G (1998) Intracranial vascular malformations. Eur Radiol 8:685–690
Ehricke HH, Schad LR, Gademann G, Wowra B, Engenhart R, Lorenz WJ (1992) Use of MR angiography for stereotactic planning. J Comput Assist Tomogr 16:35–40
Parker DL, Tsuruda JS, Goodrich KC, Alexander AL, Buswell HR (1998) Contrast-enhanced magnetic resonance angiography of cerebral arteries. A review. Invest Radiol 33:560–572
Du YP, Parker DL, Davis WL, Cao G, Buswell HR, Goodrich KC (1996) Experimental and theoretical studies of vessel contrast-to-noise ratio in intracranial time-of-flight MR angiography. J Magn Reson Imaging 6:99–108
Marchal G, Michiels J, Bosmans H, Van Hecke P (1992) Contrast-enhanced MRA of the brain. J Comput Assist Tomogr 16:25–29
Runge VM, Kirsch JE, Lee C (1993) Contrast-enhanced MR angiography. J Magn Reson Imaging 3:233–239
Jung HW, Chang KH, Choi DS, Han MH, Han MC (1995) Contrast-enhanced MR angiography for the diagnosis of intracranial vascular disease: optimal dose of gadopentetate dimeglumine. Am J Roentgenol 165:1251–1255
Sanelli PC, Mifsud MJ, Zelenko N, Heier LA (2005) CT angiography in the evaluation of cerebrovascular diseases. Am J Roentgenol 184:305–312
Stancanello J, Cavedon C, Francescon P, Cerveri P, Ferrigno G, Colombo F, Perini S (2004) Development and validation of a CT-3D rotational angiography registration method for AVM radiosurgery. Med Phys 31:1363–1371
Wu J, Chen X, Shi Y, Chen S (2000) Noninvasive three-dimensional computed tomographic angiography in preoperative detection of intracranial arteriovenous malformations. Chin Med J (Engl) 113:915–920
Tanaka H, Numaguchi Y, Konno S, Shrier DA, Shibata DK, Patel U (1997) Initial experience with helical CT and 3D reconstruction in therapeutic planning of cerebral AVMs: comparison with 3D time-of-flight MRA and digital subtraction angiography. J Comput Assist Tomogr 21:811–817
Aoki S, Sasaki Y, Machida T, Hayashi N, Shirouzu I, Ohkubo T, Terahara A, Kawamoto S, Araki T, Maehara T (1998) 3D-CT angiography of cerebral arteriovenous malformations. Radiat Med 16:263–271
Royal College of Radiologists (1998) Making the best use of a Department of Radiology: guidelines for doctors, 4th edn. Royal College of Radiologists, London
EU Council Directive (1997) Health protection of individuals against the dangers of ionising radiation in relation to medical exposure. 1997/43, Euratom, 30 June
Korosec FR, Frayne R, Grist TM, Mistretta CA (1996) Time-resolved contrast-enhanced 3D MR angiography. Magn Reson Med 36:345–351
Prince MR, Yucel EK, Kaufman JA, Harrison DC, Geller SC (1993) Dynamic gadolinium-enhanced three-dimensional abdominal MR arteriography. J Magn Reson Imaging 3:877–881
Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA (2002) Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med 48:297–305
Carroll TJ (2002) The emergence of time-resolved contrast-enhanced MR imaging for intracranial angiography. Am J Neuroradiol 23:346–348
Shim YW, Chung TS, Kang WS, Joo JY, Strecker R, Hennig J (2002) Non-invasive follow-up evaluation of post-embolized AVM with time-resolved MRA: a case report. Korean J Radiol 3:271–275
Leclerc X, Khalil C, Silvera S, Gauvrit JY, Bracard S, Meder JF, Pruvo JP (2003) Imaging of non-traumatic intracerebral hematoma. J Neuroradiol 30:303–316
Mori H, Aoki S, Masumoto T, Yoshikawa T, Tago M, Shin M, Ohtomo K, Kabasawa H (2002) Two-dimensional magnetic resonance digital substraction angiography using array spatial sensitivity encoding techniques in the assessment of intracranial hemodynamics. Radiat Med 20:223–229
Sodickson DK, McKenzie CA, Li W, Wolff S, Manning WJ, Edelman RR (2000) Contrast-enhanced 3D MR angiography with simultaneous acquisition of spatial harmonics: a pilot study. Radiology 217:284–289
Gauvrit JY, Leclerc X, Oppenheim C, Munier T, Trystram D, Rachdi H, Nataf F, Pruvo JP, Meder JF (2005) Three-dimensional dynamic MR digital subtraction angiography using sensitivity encoding for the evaluation of intracranial arteriovenous malformations: a preliminary study. Am J Neuroradiol 26:1525–1531
Paschal CB, Morris HD (2004) K-space in the clinic. J Magn Reson Imaging 19:145–159
Frayne R, Grist TM, Swan JS, Peters DC, Korosec FR, Mistretta CA (2000) 3D MR DSA: effects of injection protocol and image masking. J Magn Reson Imaging 12:476–487
Guo WY, Lindquist C, Karlsson B, Kihlstrom L, Steiner L (1993) Gamma knife surgery of cerebral arteriovenous malformations: serial MR imaging studies after radiosurgery. Int J Radiat Oncol Biol Phys 25:315–323
Kihlstrom L, Guo WY, Karlsson B, Lindquist C, Lindqvist M (1997) Magnetic resonance imaging of obliterated arteriovenous malformations up to 23 years after radiosurgery. J Neurosurg 86:589–593
Friedman WA, Bova FJ, Mendenhall WM (1995) Linear accelerator radiosurgery for arteriovenous malformations: the relationship of size to outcome. J Neurosurg 82:180–189
Lunsford LD, Kondziolka D, Flickinger JC, Bissonette DJ, Jungreis CA, Maitz AH, Horton JA, Coffey RJ (1991) Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg 75:512–524
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gauvrit, JY., Oppenheim, C., Nataf, F. et al. Three-dimensional dynamic magnetic resonance angiography for the evaluation of radiosurgically treated cerebral arteriovenous malformations. Eur Radiol 16, 583–591 (2006). https://doi.org/10.1007/s00330-005-0011-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-0011-6