Abstract
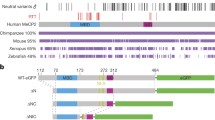
Rett syndrome is a dominant neurological disorder caused by loss-of-function mutations of methyl-CpG-binding protein 2 (MeCP2). MeCP2 is an abundant chromatin-associated protein that contains two well characterized domains. Through an N-terminal domain it recognizes methyl-CpGs and binds to nonmethylated DNA. A domain in the middle of the protein can act as a transcriptional repressor in transient transfection studies. The C-terminal region of the protein is equally essential for the function of MeCP2, as documented by recurrently found frameshift mutations. However, little is known about its functional role. Here we mapped a domain within MeCP2 capable of binding specifically to Group II WW domains of splicing factors formin-binding protein (FBP) 11 and HYPC. Binding was assessed by glutathione S-transferase pull-down assays and coimmunoprecipitation assays. The Group II WW domain binding region was localized from residue 325 to the C-terminus, with the interacting proline-rich sequence at its center. We then used comparison with genotype-phenotype studies in Rett syndrome patients to evaluate the relevance of Group II WW domain interactions of MeCP2 for pathogenesis. Truncation of the WW domain binding region by 48 C-terminal amino acids (to residue 438), causing Rett syndrome, resulted in reduced or loss of WW domain binding activity. Truncation to residue 400, representing a large group of frameshift mutations accounting for approx. 10% of Rett syndrome cases, abolished WW domain binding activity completely. On the other hand, two benign missense mutations did not affect binding. Furthermore, a short C-terminal truncation and an internal deletion, both causing mild to moderate mental retardation in males, were associated with weak or loss of WW domain binding activity.


Similar content being viewed by others
Abbreviations
- CR :
-
Conserved region
- FBP :
-
Formin-binding protein
- GST :
-
Glutathione S-transferase
- HA :
-
Hemagglutinin
- HEK :
-
Human embryonic kidney
- MeCP2 :
-
Methyl-CpG-binding protein 2
- PMSF :
-
Phenylmethylsulfonyl fluoride
- WDR :
-
Group II WW domain binding region
References
Hendrich B, Bird A (1998) Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 18:6538–6547
Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A (1992) Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69:905–914
Kries JP von, Buhrmester H, Strätling WH (1991) A matrix/scaffold attachment region binding protein: identification, purification, and mode of binding. Cell 64:123–135
Weitzel JM, Buhrmester H, Strätling WH (1997) Chicken MAR-binding protein ARBP is homologous to rat methyl-CpG-binding protein MeCP2. Mol Cell Biol 17:5656–5666
Wakefield RID, Smith BO, Nan X, Free A, Soteriou A, Uhrin D, Bird AP, Barlow PN (1999) The solution structure of the domain from MeCP2 that binds to methylated DNA. J Mol Biol 291:1055–1065
Heitmann B, Maurer T, Weitzel JM, Strätling WH, Kalbitzer HR, Brunner E (2003) Solution structure of the matrix attachment region-binding domain of chicken MeCP2. Eur J Biochem 270:1–8
Nan X, Campoy J, Bird A (1997) MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88:471–481
Nan X, Ng H-H, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386–389
Jones PL, Veenstra GJC, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19:187–191
Yu F, Thiesen J, Strätling WH (2000) Histone deacetylase-independent transcriptional repression by methyl-CpG-binding protein 2. Nucleic Acids Res 28:2201–2206
Rett A (1966) Über ein eigenartiges hirnatrophisches Syndrom bei Hyperammonämie im Kindesalter. Wien Med Wochenschr 116:723–726
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188
Baumann ML, Kemper TL, Arin DM (1995) Microscopic observations of the brain in Rett syndrome. Neuropediatrics 26:105–108
Belichenko PV, Oldfors A, Hagberg B, Dahlström A (1994) Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport 5:1509–1513
Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY (2002) Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlates with neuronal maturation. Hum Mol Genet 11:115–124
Chen RZ, Akbarian S, Tudor M, Jaenisch R (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27:327–331
Colantuoni C, Jeon O-H, Hyder K, Chenchik A, Khimani AH, Narayanan V, Hoffmann EP, Kaufmann WE, Naidu S, Pevsner J (2001) Gene expression profiling in postmortem Rett syndrome brain: differential gene expression and patient classification. Neurobiol Dis 8:847–865
Dragich J, Houwink-Manville I, Schanen C (2000) Rett syndrome: a surprising result of mutation in MECP2. Hum Mol Genet 9:2365–2375
Chandler SP, Guschin D, Landsberger N, Wolffe AP (1999) The methyl-CpG binding transcriptional repressor MeCP2 stably associates with nucleosomal DNA. Biochemistry 38:7008–7018
Macias MJ, Wiesner S, Sudol M (2002) WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett 513:30–37
Bedford MT, Sarbassova D, Xu J, Leder P, Yaffe MB (2000) A novel Pro-Arg motif recognized by WW domains. J Biol Chem 275:10359–10369
Chan DC, Leder P (1996) Genetic evidence that formins function within the nucleus. J Biol Chem 271:23472–23477
Morris DP, Greenleaf AL (2000) The splicing factor, Prp40, binds the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem 275:39935–39943
Bedford MT, Chan DC, Leder P (1997) FBP WW domains and the Abl SH3 domain bind to a specific class of proline-rich ligands. EMBO J 16:2376–2383
Chan DC, Bedford MT, Leder P (1996) Formin binding proteins bear WWP/WW domains that bind proline-rich peptides and functionally resemble SH3 domains. EMBO J 15:1045–1054
Reichwald K, Thiesen J, Wiehe T, Weitzel J, Strätling WH, Kioschis P, Poustka A, Rosenthal A, Platzer M (2000) Comparative sequence analysis of the MECP2-locus in human and mouse reveals new transcribed regions. Mamm Genome 11:182–190
Wang W, Malcolm BA (1999) Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. Biotechniques 26:680–682
Passani LA, Bedford MT, Faber PW, McGinnis KM, Sharp AH, Gusella JF, Vonsattel J-P, MacDonald ME (2000) Huntingtin’s WW domain partners in Huntington’s disease post-mortem brain fulfill genetic criteria for direct involvement in Huntington’s disease pathogenesis. Hum Mol Genet 9:2175–2182
Espanel X, Sudol M (1999) A single point mutation in a Group I WW domain shifts its specificity to that of Group II domains. J Biol Chem 274:17284–17289
Bedford MT, Reed R, Leder P (1998) WW domain-mediated interactions reveal a spliceosome-associated protein that binds a third class of proline-rich motif: the proline glycine and methionine-rich motif. Proc Natl Acad Sci USA 95:10602–10607
Buyse IM, Fang P, Hoon KT, Amir RE, Zoghbi HY, Roa BB (2000) Diagnostic testing for Rett syndrome by DHPLC and direct sequencing analysis of the MECP2 gene: identification of several novel mutations and polymorphisms. Am J Hum Genet 67:1428–1436
Wan M, Lee SSJ, Zhang X, Houwink-Manville I, Song H-R, Amir RE, Budden S, Naidu S, Pereira JLP, Lo IFM et al (1999) Am J Hum Genet 65:1520–1529
Couvert P, Bienvenu T, Aquaviva C, Poirier K, Moraine C, Gendrot C, Verloes A, Andrès C, Le Fevre AC, Souville I et al (2001) MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet 10:941–946
Laccone F, Zoll B, Huppke P, Hanefeld F, Pepinski W, Trappe R (2002) MECP2 gene nucleotide changes and their pathogenicity in males: proceed with caution. J Med Genet 39:586–588
Trappe R, Laccone F, Cobilanchi J, Meins M, Huppke P, Hanefeld F, Engel W (2001) MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin. Am J Hum Genet 68:1093–1101
Kleefstra T, Yntema HG, Oudakker AR, Romein T, Sistermans E, Nillessen W, van Bokhoven H, de Vries BBA, Hamel BCJ (2002) De novo MECP2 frameshift mutation in a boy with moderate mental retardation, obesity and gynaecomastia. Clin Genet 61:359–362
Yntema HG, Oudakker AR, Kleefstra T, Hamel BCJ, van Bokhoven H, Chelly J, Kalscheuer VM, Fryns J-P, Raynaud M, Moizard M-P, Moraine C (2002) In-frame deletion in MECP2 causes mild nonspecific mental retardation. Am J Med Genet 107:81–83
Macias MJ, Hyvonen M, Baraldi E, Schultz J, Sudol M, Saraste M, Oschkinat H (1996) Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature 382:646–649
Kerr TP, Sewry CA, Robb SA, Roberts RG (2001) Long mutant dystrophins and variable phenotypes: evasion of nonsense-mediated decay? Hum Genet 109:402–407
Huppke P, Held M, Hanefeld F, Engel W, Laccone F (2002) Influence of mutation type and location on phenotype in 123 patients with Rett syndrome. Neuropediatrics 33:63–68
Zappella M, Meloni I, Longo I, Hayek G, Renieri A (2001) Preserved speech variants of the Rett syndrome: molecular and clinical analysis. Am J Med Genet 104:14–22
Kao H-Y, Siliciano PG (1996) Identification of Prp40, a novel essential yeast splicing factor associated with the U1 small nuclear ribonucleoprotein particle. Mol Cell Biol 16:960–967
Abovich N, Rosbash M (1997) Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell 89:403–412
Tudor M, Akbarian S, Chen RZ, Jaenisch R (2002) Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci USA 99:15536–15541
Acknowledgements
We gratefully acknowledge M.T. Bedford’s generous gift of plasmid DNAs. We also thank S. Cloppenburg for plasmid constructions, S. Giehler for excellent technical assistance, and C. Stocking and M. Harbers for careful reading of the manuscript. This work was supported by grants (to W.H.S.) from the Deutsche Forschungsgemeinschaft (Str 145/12-3 and SFB 545/B2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buschdorf, J.P., Strätling, W.H. A WW domain binding region in methyl-CpG-binding protein MeCP2: impact on Rett syndrome. J Mol Med 82, 135–143 (2004). https://doi.org/10.1007/s00109-003-0497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-003-0497-9