Abstract
Rett syndrome is a severe neurodevelopmental disorder that arises from mutations in the X-linked MECP2 gene. It is almost exclusively seen in girls due to the predominant occurrence of the mutations on the paternal X-chromosome, and also the early postnatal lethal effect of the disease causing mutations in hemizygous boys. We identified a boy with features of classic Rett syndrome who is mosaic for the truncating MECP2 mutation R270X. Chromosome analysis showed normal karyotype. These results indicate that a MECP2 mutation associated with Rett syndrome in females could lead to a similar phenotype in males as a result of somatic mosaicism.
Similar content being viewed by others
Introduction
Classical Rett syndrome (RTT-MIM 312750) was first defined as a neurodevelopmental disorder that is primarily seen in girls.1,2 Following the hypothesis of X-linked dominant mutations that are lethal in hemizygous males,2 the RTT gene was localised to Xq28 by exclusion mapping studies in rare familial cases.3 Subsequently, MECP2 (methyl-CpG-binding protein 2) was identified as the gene responsible for this disorder by a systematic search for mutations in genes from the Xq28 region in RTT patients.4 Normal development until 6–18 months of age, followed by gradual loss of speech and purposeful hand use, microcephaly, seizures, ataxia, and stereotypic hand movements are among the cardinal features of Rett syndrome.5 Femaleness is also included in the above-mentioned diagnostic criteria.
A wide range of MECP2 mutations has been identified in Rett syndrome patients.6 Interestingly, mutations of the same gene have been documented in male patients affected by severe encephalopathy,3,6 and X-linked mental retardation.7 One boy affected with a nonfatal neurodevelopmental disorder with similarities to Rett syndrome was found to be mosaic for a MECP2 gene mutation.8 In addition, an Angelman syndrome-like phenotype has recently been documented in girls with MECP2 mutations.9
We and others have observed boys with clinical features of Rett syndrome and a normal male karyotype.10,11,12 Several cases of 47,XXY males with a clinical diagnosis of Rett syndrome have been reported, although to our knowledge only one has been reported so far as having a MECP2 mutation.13 In order to determine whether MECP2 is involved in the development of the disease phenotype in males, we performed mutation screening in a patient not reported before, and diagnosed as Rett syndrome.
Materials and methods
Case report
BF was the product of a non-eventful pregnancy born to a 40-year-old father and 29-year-old mother. He has two brothers (15 and 17 years old), and one sister (18 years old), who are all healthy. His birth weight was 3500 g. Although he appeared to be a hypotonic child, he had normal psychomotor development through the first 6 months. Head control was achieved during the first 4 months. He sat with support at age 5 months, and without support at 12 months. He rolled over, transferred toys from one hand to the other, and babbled nonspecifically at around 6–8 months. He was also able to bring the milk bottle to his mouth alone. Loss of acquired purposeful hand skills began around 11 months, and stereotypic hand movements such as patting, squeezing became apparent at 15 months. He never crawled or walked. He has never spoken. None of the exclusion criteria (evidence of intrauterine growth retardation, organomegaly, retinopathy, microcephaly at birth etc.) was ever present. We first saw him at 2 years of age, and a tentative diagnosis of Rett syndrome was made. On examination at 12 years of age (Figure 1, Table 1), he was microcephalic with a head circumference of 47 cm (below second percentile), and brachycephalic with titubation of the head. His height was 110 cm and weight 18 kg, which are both below the third percentile. Sialorrhea and bruxism were present. Stereotypic hand movements, tremors, and apraxia were noted. Deep tendon reflexes were increased bilaterally, and the clonus and Babinski signs were positive. His parents describe BF as a happy child who likes listening to music. No breath holding or abnormal skin pigmentation was observed. He had a thoracic scoliosis and poor lower-limb musculature. His hands and feet were small, and cold. Examination of the genitalia revealed presence of hypospadias and cryptorchidism. He experienced a total of three febrile seizures at the ages of five, 11, and 15 months, which was treated with phenobarbital for a short period of time. Currently he is not on this medication.
Electroencephalography showed excess of slow-wave activity and paroxysmal sharp theta wave activity prominent on wake recordings of frontal regions. A magnetic resonance scan at age 12 was normal. Based on these findings, RTT currently at stage IV was considered. Therefore, we obtained genomic DNA isolated from venous blood and hair root samples. Informed consent was obtained from each subject.
Genotyping
As a first step of the MECP2 gene screening procedure, we looked for eight recurrent mutations (R106W, P152R, T158M, R168X, R255X, R270X, R288X, and R306C) by PCR, and restriction enzyme digestion,4,6 which was further confirmed by sequencing. Two different sets of primers were used for the detection of the R270X mutation. The first set is 5′-GGC AGG AAG CGA AAA GCT GAG-3′ and 5′-TGA GTG GTG GTG ATG GTG GTG GG-3′ which yields a 366 bp fragment. The second set of primers which amplify a 380 bp fragment was used to confirm the results: 5′AAC CAC CTA AGA AGC CAA A-3′ and 5′-CTG CAC AGA TCG GAT AGA AGA C-3′. All reactions were performed in a total volume of 25 μl, with 10× buffer, 1.5 U Taq polymerase, 1.5 mM MgCl2, 200 μM dNTP, and 10 pmol of each primer. PCR conditions were as follows: 95°C for 3 min, 30 cycles of 30 s at 94°C, 58°C and 30 s at 72°C. Ten minutes of 72°C was added at the extension step.
Results
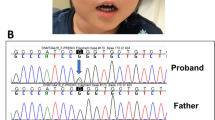
We identified the truncating mutation 808C→T (R270X) in BF's DNA sample (Figure 2A). The same mutation was present also in two girls diagnosed with RTT.14 Interestingly, the mutant allele in the affected boy was present along with the wild-type allele though at a reduced dosage when compared with the samples of the two girls. Whereas the mutant (T) and the wild-type (C) nucleotide peaks were equal in the two girls, the T allele peak was smaller in the boy. In order to quantitate the dosage of the alleles, genomic DNA samples of the boy obtained from three independent blood samplings done at different times, and from one of the girls was re-amplified with the same primers used in the sequencing reactions, and then subjected to restriction endonuclease treatment since the mutation abolishes an NlaIV restriction site. Densitometric scanning of the restriction fragments revealed that the T : C allele ratio is approximately 56 : 44 in the girl and approximately 36 : 64 in the boy (Figure 2B). Furthermore, hair root samples of BF was obtained for DNA isolation. Three independent samples were subjected to PCR amplification and restriction endonuclease treatment as indicated above. The results of these experiments also revealed the presence of somatic mosaicism with the T : C allele ration of 41 : 59. Since the restriction digestion method described above relies on the absence of digestion of the mutated allele, but does not include a control for complete digestion of the DNA, we amplified a new 380 bp fragment, which contains four constant NlaIV sites in addition to the sequences encompassing the R270X mutation. Digestion of the PCR products obtained from BF's DNA sample, and the positive control sample revealed the presence of a mutation specific 177 bp fragment in addition to the constant fragments of 91, 86, 80, 74, and 49 bp (Figure 2C). The 177 bp fragment was absent in the normal control sample. This result confirmed the presence of the R270X mutation in BF and the positive control sample. Chromosome analysis on blood revealed a normal 46,XY karyotype. These results suggest that the boy is a somatic mosaic for the R270X mutation.
Analysis of the recurrent MECP2 mutation R270X. (A) Sequence analysis of patients 00–196 (BF), and 99–104 (female RTT) who are both heterozygous for the mutation R270X (808C→T). Recognition site of the restriction enzyme NlaIV is abolished in the presence of the mutation indicated by an arrow. (B) Restriction digestion of samples 99–104 (lane 1), 00–196hr (hair root) (lane 2), and 00–196 (lane 3) with NlaIV after amplification with the first set of primers results in a pattern with fragments 370 bp (mutant allele) and 304 bp+66 bp (normal allele) in length, indicating presence of the mutant and wild type alleles for the R270X mutation in the patient samples. Results of the densitometric scans for the wild type alleles and the mutant allele in samples 00–196 and 99–104 is indicated below the individual lanes. The values represent the means of three independent experiments, with ranges in parenthesis. Calculations were done using the Multi-Analyst software version 1.1 (Bio-Rad Laboratories). (C) Restriction digestion of the samples 00–196 (lane 1), 99–104 (lane 2), and a female control 99–91 (lane 3) with NlaIV after amplification with the second set of primers results in a pattern with fragments 177, 80, 74, 49 bp (mutant allele) and 91, 86, 80, 74, and 49 bp (normal allele) in length, indicating presence of the mutant and wild type alleles for the R270X mutation in the patient samples. M stands for the size marker pUC Mix Marker, 8 (fragments of 501/489, 404, 331, 242, 190, 147, and 111/110 bp are shown in B, and fragments of 242, 190, 147, 111/110, and 67 bp are shown in C).
Discussion
So far, MECP2 mutations have been shown to be associated with Rett syndrome in females, predominantly as de novo mutations on the paternal X-chromosome;15 neonatal encephalopathy6 or X-linked non-specific mental retardation7 in males which is transmitted through the maternal X-chromosome; X-linked non-specific mental retardation in females;7 and finally Rett syndrome in XY males when the mutation is present in cells alongside a normal cell line,8 or in XXY males.
Somatic mosaicism for the R270X mutation in BF could be the result of an early post-zygotic mutation or chimerism. We think chimerism – which is defined as the coexistence of different cell populations of more than one zygote in one body – is less likely since whole body chimeras result from fertilisation of one or more egg nuclei by two sperms, and is often associated with 46,XY/46,XX karyotype.16 BF's karyotype analysis did not show a chromosomal abnormality. Analysis of highly polymorphic markers used in DNA profiling for individual identification would provide the definitive answer for the post-zygotic mutation vs chimerism issue since one would expect to see the inheritance of two alleles of a locus from one parent in addition to the allele from the other parent if enough loci are studied in a chimeric individual.
Based on the function of the MECP2 gene abnormal epigenetic regulation has been proposed as the mechanism underlying the pathogenesis of RTT.4 DNA methylation-dependent silencing which is a known mechanism for maintenance of X-chromosome inactivation (XCI) and gametic imprinting are good candidates to study for abnormalities in individuals with RTT.6 In this model, escape from XCI causing biallelic overexpression of X-linked genes and/or misexpression of imprinted genes is expected. However, with the observation of a male RTT patient who is mosaic for a MECP2 mutation, disturbances in the expression of genes subject to XCI as a causative mechanism for RTT becomes unlikely since males are not subject to X-inactivation yet they display the RTT phenotype.
Somatic mosaicism has been documented in various genetic diseases including X-linked disorders in both males and females. These include ornithine transcarbamylase deficiency,17 Duchenne muscular dystrophy,18 and hemophilia A.19 Although it is not clear whether the single-gene mutation occurred as a postzygotic event or at the half-chromatid stage, before fertilisation,20 somatic mosaicism should be considered when an X-linked dominant disease is observed in a male.
References
Rett A . Uber ein eigenartiges hirnatrophisches Syndrom bei Hyperammonemie im Kindesalter Wien Med Wochenschr 1966 116: 723–738
Hagberg B, Aicardi J, Dias K, Ramos O . A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases Ann Neurol 1983 14: 471–479
Schanen NC, Francke U . A severely affected male born into a Rett syndrome kindred supports X-linked inheritance and allows extension of the exclusion map Am J Hum Genet 1998 63: 267–269
Amir RE, Van den Veyver IB, Wan M et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2 Nat Genet 1999 23: 185–188
Trevathan E, Naidu S . The clinical recognition and differential diagnosis of Rett syndrome J Child Neurol 1988 3 Suppl: S6–S16
Wan M, Sung Jae Lee S, Zhang X et al. Rett Syndrome and Beyond: Recurrent Spontaneous and Familial MECP2 Mutations at CpG Hotspots Am J Hum Genet 1999 65: 1520–1529
Meloni I, Bruttini M, Longo I et al. A mutation in the Rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males Am J Hum Genet 2000 67: 982–985
Clayton-Smith J, Watson P, Ramsden S, Black GCM . Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males Lancet 2000 356: 830–832
Watson P, Black G, Ramsden S et al. Angelman syndrome phenotype associated with mutations in MECP2, a gene encoding a methyl CpG binding protein J Med Genet 2001 38: 224–228
Philippart M . The Rett syndrome in males Brain Dev 1990 12: 33–36
Eeg-Olofsson O, Al-Zuhair AGH, Teebi AS et al. A boy with Rett syndrome? Brain Dev 1990 12: 529–532
Topçu M, Topaloglu H, Renda Y et al. The Rett syndrome in males Brain Dev 1991 13: 62
Hoffbuhr K, Devaney JM, LaFleur B et al. MeCP2 mutations in children with and without the phenotype of Rett syndrome Neurology 2001 56: 1489–1495
Akyerli C, Sayi A, Kocoglu RS, Cimbis M, Topcu M, Ozcelik T . Analysis of MECP2 gene mutations in Turkish Rett syndrome patients Eur J Hum Genet 2001 9 suppl. 1: P1450
Trappe R, Laccone F, Cobilanschi J et al. MECP2 mutations in sporadic cases of Rett syndrome are almost exclusively of paternal origin Am J Hum Genet 2001 68: 1093–1101
Repas-Humpe LM, Humpe A, Lynen R et al. A dispermic chimerism in a 2-year-old Caucasian boy Ann Hematol 1999 78: 431–434
Maddalena A, Sosnoski DM, Berry GT, Nussbaum RL . Mosaicism for an intragenic deletion in a boy with mild ornithine transcarbamylase deficiency New Engl J Med 1988 319: 999–1003
Bakker E, Veenema H, Den Dunnen JT et al. Germinal mosaicism increases the recurrence risk for “new” Duchenne muscular dystropy mutations J Med Genet 1989 26: 553–559
Leuer M, Oldenburg J, Levergne J-M et al. Somatic mosaicism in hemophilia: A fairly common event Am J Hum Genet 2001 69: 75–87
Gartler SM, Francke U . Half Chromatid Mutations: Transmission in Humans? Am J Hum Genet 1975 27: 218–223
Kerr AM, Nomura Y, Armstrong D et al. Guidelines for reporting different clinical features in cases with MECP2 mutations Brain Dev 2001 23: 208–211
Acknowledgements
We would like to express our gratitude to the family for their help with this project. We thank Dr Uta Francke for critical reading of the manuscript, Drs Ergül Tunçbilek and Dilek Aktaş for performing the cytogenetic analysis, Dr Göknur Haliloğlu for help in obtaining patient samples, and Emre Sayan, Tolga Çağatay, Tülay Arayıcı for dosage analysis experiments and DNA sequencing. This work was supported by Bilkent University, and TÜBİTAK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Topçu, M., Akyerli, C., Sayı, A. et al. Somatic mosaicism for a MECP2 mutation associated with classic Rett syndrome in a boy. Eur J Hum Genet 10, 77–81 (2002). https://doi.org/10.1038/sj.ejhg.5200745
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5200745