Key Points
-
MicroRNAs (miRNAs) are a large family of post-transcriptional regulators of gene expression that control many developmental and cellular processes in eukaryotic organisms. Recent research indicates that miRNA regulators themselves are subject to sophisticated control at the levels of miRNA metabolism and function.
-
Transcription of miRNA genes is regulated similarly to that of protein-coding genes, and is a major level of control responsible for tissue-specific or development-specific expression. miRNAs are uniquely suited to participate in autoregulatory feedback circuits owing to their potential to directly repress mRNAs that encode factors involved in miRNA synthesis.
-
miRNA precursors are processed to mature miRNAs in two steps involving the RNase III family enzymes Drosha and Dicer. These maturation steps are subjects of intricate regulation (either positive or negative) by protein factors that either interact with Drosha or Dicer, or bind to miRNA precursors. Many regulators affect the processing of a broad range of miRNAs, indicating that they can modulate expression of entire gene networks.
-
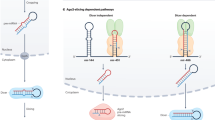
Many miRNA precursors undergo editing by adenosine deaminases that catalyse the conversion of adenosine to inosine in dsRNA segments, altering the base-pairing and structural properties of transcripts. These modifications can affect miRNA processing and can also change properties of mature miRNAs.
-
miRNAs function in association with argonaute and glycine-tryptophan protein of 182 kDa (GW182) proteins, which are the main components of the miRNA-induced silencing complex (miRISC). As part of the miRISC, miRNAs base-pair to target mRNAs and induce their translational repression or deadenylation and degradation.
-
miRISCs interact with many additional factors that are required for miRNA function or for modulation. The mode of action for most of the accessory proteins remains unknown. Argonaute and GW182 proteins, and also some accessory factors, are subject to post-translational modifications that may regulate their activity.
-
The activity of miRISCs interacting with the mRNA 3′-UTR can be modulated by RNA-binding proteins interacting with the same mRNA. The same RNA-binding protein can, depending on the mRNA or cellular context, either prevent or activate miRISC repression.
-
It is generally thought that miRNAs are highly stable molecules. However, in some cell types, in particular in neurons, miRNAs decay very rapidly and their turnover is dependent on neuronal activity. The stability of mature miRNAs may be regulated by the untemplated addition of adenosine or uracil residues to the miRNA 3′ end.
-
miRNA repression may involve specific cellular structures or compartments such as processing bodies or multivesicular bodies. In neurons, selected miRNAs are enriched at distal sites in dendrites, and evidence exists to suggest that synaptic stimulation is accompanied by reactivation of mRNAs targeted by miRNAs at dendritic spines.
Abstract
MicroRNAs (miRNAs) are a large family of post-transcriptional regulators of gene expression that are ∼21 nucleotides in length and control many developmental and cellular processes in eukaryotic organisms. Research during the past decade has identified major factors participating in miRNA biogenesis and has established basic principles of miRNA function. More recently, it has become apparent that miRNA regulators themselves are subject to sophisticated control. Many reports over the past few years have reported the regulation of miRNA metabolism and function by a range of mechanisms involving numerous protein–protein and protein–RNA interactions. Such regulation has an important role in the context-specific functions of miRNAs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Carthew, R. W. & Sontheimer, E. J. Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009).
Chekulaeva, M. & Filipowicz, W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 21, 452–460 (2009).
Fabian, M. R., Sonenberg, N. & Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 (2010).
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nature Rev. Mol. Cell Biol. 10, 126–139 (2009).
Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 136, 669–687 (2009).
Davis, B. N. & Hata, A. Regulation of microRNA biogenesis: a miRiad of mechanisms. Cell Commun. Signal 7, 18 (2009).
Turner, M. J. & Slack, F. J. Transcriptional control of microRNA expression in C. elegans: promoting better understanding. RNA Biol. 6, 49–53 (2009).
Kim, J. et al. A microRNA feedback circuit in midbrain dopamine neurons. Science 317, 1220–1224 (2007).
Johnston, R. J. Jr, Chang, S., Etchberger, J. F., Ortiz, C. O. & Hobert, O. MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc. Natl Acad. Sci. USA 102, 12449–12454 (2005). Identified a double-negative feedback circuit between miRNAs and transcription factors that is essential for neuronal cell fate determination.
Stark, A. et al. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands. Genes Dev. 22, 8–13 (2008).
Tyler, D. M. et al. Functionally distinct regulatory RNAs generated by bidirectional transcription and processing of microRNA loci. Genes Dev. 22, 26–36 (2008).
Han, J. et al. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 136, 75–84 (2009).
Triboulet, R., Chang, H. M., Lapierre, R. J. & Gregory, R. I. Post-transcriptional control of DGCR8 expression by the Microprocessor. RNA 15, 1005–1011 (2009). References 13 and 14 identified reciprocal co-regulation between Drosha and DGCR8 proteins.
Gregory, R. I. et al. The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 (2004).
Faller, M., Matsunaga, M., Yin, S., Loo, J. A. & Guo, F. Heme is involved in microRNA processing. Nature Struct. Mol. Biol. 14, 23–29 (2007).
Chendrimada, T. P. et al. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744 (2005).
Melo, S. A. et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nature Genet. 41, 365–370 (2009).
Paroo, Z., Ye, X., Chen, S. & Liu, Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell 139, 112–122 (2009). Established the link between a major cell signalling pathway and the miRNA machinery, showing that mitogenic stimulation mediated by MAPK/ERK is associated with TRBP phosphorylation and leads to upregulation of growth-promoting miRNAs.
Forman, J. J., Legesse-Miller, A. & Coller, H. A. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc. Natl Acad. Sci. USA 105, 14879–14884 (2008).
Davalos, V. & Esteller, M. MicroRNAs and cancer epigenetics: a macrorevolution. Curr. Opin. Oncol. 22, 35–45 (2010).
Ma, E., MacRae, I. J., Kirsch, J. F. & Doudna, J. A. Autoinhibition of human dicer by its internal helicase domain. J. Mol. Biol. 380, 237–243 (2008).
Lugli, G., Larson, J., Martone, M. E., Jones, Y. & Smalheiser, N. R. Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J. Neurochem. 94, 896–905 (2005).
Zhang, H., Kolb, F. A., Brondani, V., Billy, E. & Filipowicz, W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 21, 5875–5885 (2002).
Nakagawa, A., Shi, Y., Kage-Nakadai, E., Mitani, S. & Xue, D. Caspase-dependent conversion of Dicer ribonuclease into a death-promoting deoxyribonuclease. Science 328, 327–334 (2010).
Chiang, H. R. et al. Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev. 24, 992–1009 (2010).
Frank, F., Sonenberg, N. & Nagar, B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465, 818–822 (2010).
Krol, J. et al. Structural features of microRNA (miRNA) precursors and their relevance to miRNA biogenesis and small interfering RNA/short hairpin RNA design. J. Biol. Chem. 279, 42230–42239 (2004).
Ro, S., Park, C., Young, D., Sanders, K. M. & Yan, W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Res. 35, 5944–5953 (2007).
Winter, J., Jung, S., Keller, S., Gregory, R. I. & Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nature Cell Biol. 11, 228–234 (2009).
Viswanathan, S. R. & Daley, G. Q. Lin28: A microRNA regulator with a macro role. Cell 140, 445–459 (2010).
Rybak, A. et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nature Cell Biol. 10, 987–993 (2008).
Lehrbach, N. J. et al. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nature Struct. Mol. Biol. 16, 1016–1020 (2009).
Hagan, J. P., Piskounova, E. & Gregory, R. I. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nature Struct. Mol. Biol. 16, 1021–1025 (2009).
Heo, I. et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol. Cell 32, 276–284 (2008). Describes uridylation of pre-let-7 mediated by LIN-28. References 33, 36 and 37 identified TUTases involved in this process.
Heo, I. et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 (2009).
Jones, M. R. et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nature Cell Biol. 11, 1157–1163 (2009).
Viswanathan, S. R., Daley, G. Q. & Gregory, R. I. Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008).
Roush, S. & Slack, F. J. The let-7 family of microRNAs. Trends Cell Biol. 18, 505–516 (2008).
Viswanathan, S. R. et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nature Genet. 41, 843–848 (2009).
Fukuda, T. et al. DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nature Cell Biol. 9, 604–611 (2007).
Suzuki, H. I. et al. Modulation of microRNA processing by p53. Nature 460, 529–533 (2009).
Davis, B. N., Hilyard, A. C., Lagna, G. & Hata, A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454, 56–61 (2008).
Yu, B. et al. The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc. Natl Acad. Sci. USA 105, 10073–10078 (2008).
Bracken, C. P. et al. Regulation of cyclin D1 RNA stability by SNIP1. Cancer Res. 68, 7621–7628 (2008).
Pawlicki, J. M. & Steitz, J. A. Nuclear networking fashions pre-messenger RNA and primary microRNA transcripts for function. Trends Cell Biol. 20, 52–61 (2010).
Gruber, J. J. et al. Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell 138, 328–339 (2009).
Sabin, L. R. et al. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell 138, 340–351 (2009).
Laubinger, S. et al. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 105, 8795–8800 (2008).
Wu, H. et al. A splicing-independent function of SF2/ASF in microRNA processing. Mol. Cell 38, 67–77 (2010).
Michlewski, G., Guil, S., Semple, C. A. & Caceres, J. F. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol. Cell 32, 383–393 (2008).
Trabucchi, M. et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 459, 1010–1014 (2009). References 51 and 52 identified regulators that bind to miRNA precursors and stimulate their processing.
Scadden, A. D. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nature Struct. Mol. Biol. 12, 489–496 (2005).
Kawahara, Y., Zinshteyn, B., Chendrimada, T. P., Shiekhattar, R. & Nishikura, K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer–TRBP complex. EMBO Rep. 8, 763–769 (2007).
Kawahara, Y. et al. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 36, 5270–5280 (2008).
Heale, B. S. et al. Editing independent effects of ADARs on the miRNA/siRNA pathways. EMBO J. 28, 3145–3156 (2009).
Kawahara, Y. et al. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science 315, 1137–1140 (2007). Describes a change in targeting specificity of miRNA induced by pre-miRNA editing.
Ghildiyal, M., Xu, J., Seitz, H., Weng, Z. & Zamore, P. D. Sorting of Drosophila small silencing RNAs partitions microRNA* strands into the RNA interference pathway. RNA 16, 43–56 (2010).
Czech, B. et al. Hierarchical rules for Argonaute loading in Drosophila. Mol. Cell 36, 445–456 (2009).
Okamura, K., Liu, N. & Lai, E. C. Distinct mechanisms for microRNA strand selection by Drosophila Argonautes. Mol. Cell 36, 431–444 (2009).
Iwasaki, S., Kawamata, T. & Tomari, Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol. Cell 34, 58–67 (2009).
Su, H., Trombly, M. I., Chen, J. & Wang, X. Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev. 23, 304–317 (2009).
Azuma-Mukai, A. et al. Characterization of endogenous human Argonautes and their miRNA partners in RNA silencing. Proc. Natl Acad. Sci. USA 105, 7964–7969 (2008).
Ender, C. et al. A human snoRNA with microRNA-like functions. Mol. Cell 32, 519–528 (2008).
Hafner, M. et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141 (2010).
Cheloufi, S., Dos Santos, C. O., Chong, M. M. & Hannon, G. J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589 (2010).
Cifuentes, D. et al. A Novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328, 1694–1698 (2010).
O'Carroll, D. et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 21, 1999–2004 (2007).
Wu, L., Fan, J. & Belasco, J. G. Importance of translation and nonnucleolytic ago proteins for on-target RNA interference. Curr. Biol. 18, 1327–1332 (2008).
Diederichs, S. & Haber, D. A. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell 131, 1097–1108 (2007).
Johnston, M., Geoffroy, M. C., Sobala, A., Hay, R. & Hutvagner, G. HSP90 protein stabilizes unloaded argonaute complexes and microscopic P-bodies in human cells. Mol. Biol. Cell 21, 1462–1469 (2010).
Tahbaz, N., Carmichael, J. B. & Hobman, T. C. GERp95 belongs to a family of signal-transducing proteins and requires Hsp90 activity for stability and Golgi localization. J. Biol. Chem. 276, 43294–43299 (2001).
Qi, H. H. et al. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature 455, 421–424 (2008).
Rybak, A. et al. The let-7 target gene mouse lin-41 is a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. Nature Cell Biol. 11, 1411–1420 (2009).
Zeng, Y., Sankala, H., Zhang, X. & Graves, P. R. Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. Biochem. J. 413, 429–436 (2008).
Bhattacharyya, S. N., Habermacher, R., Martine, U., Closs, E. I. & Filipowicz, W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125, 1111–1124 (2006). This paper and reference 97 were the first studies to show that RBPs interacting with the mRNA 3′-UTR can relieve or prevent miRNA-mediated repression.
Leung, A. K. & Sharp, P. A. microRNAs: a safeguard against turmoil? Cell 130, 581–585 (2007).
Djuranovic, S. et al. Allosteric regulation of Argonaute proteins by miRNAs. Nature Struct. Mol. Biol. 17, 144–150 (2010).
Kiriakidou, M. et al. An mRNA m7G cap binding-like motif within human Ago2 represses translation. Cell 129, 1141–1151 (2007).
Eulalio, A., Huntzinger, E. & Izaurralde, E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nature Struct. Mol. Biol. 15, 346–353 (2008).
Wang, Y. et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature 461, 754–761 (2009).
Eulalio, A., Tritschler, F. & Izaurralde, E. The GW182 protein family in animal cells: new insights into domains required for miRNA-mediated gene silencing. RNA 15, 1433–1442 (2009).
Li, S. et al. Identification of GW182 and its novel isoform TNGW1 as translational repressors in Ago2-mediated silencing. J. Cell Sci. 121, 4134–4144 (2008).
Eystathioy, T. et al. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell 13, 1338–1351 (2002).
Yang, Z. et al. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J. Cell Sci. 117, 5567–5578 (2004).
Gibbings, D. J., Ciaudo, C., Erhardt, M. & Voinnet, O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nature Cell Biol. 11, 1143–1149 (2009). References 86 and 107 identified MVBs as organelles contributing to the miRISC function, possibly by promoting miRISC loading or turnover.
Hilbert, M., Karow, A. R. & Klostermeier, D. The mechanism of ATP-dependent RNA unwinding by DEAD box proteins. Biol. Chem. 390, 1237–1250 (2009).
Robb, G. B. & Rana, T. M. RNA helicase A interacts with RISC in human cells and functions in RISC loading. Mol. Cell 26, 523–537 (2007).
Tomari, Y. et al. RISC assembly defects in the Drosophila RNAi mutant armitage. Cell 116, 831–841 (2004).
Hammell, C. M., Lubin, I., Boag, P. R., Blackwell, T. K. & Ambros, V. nhl-2 modulates microRNA activity in Caenorhabditis elegans. Cell 136, 926–938 (2009). References 74 and 90–92 identified the TRIM-NHL family of ubiquitin ligases as miRNA regulators in mammals, flies and worms. They also provide evidence that different TRIM-NHL proteins may act through different mechanisms.
Neumuller, R. A. et al. Mei-P26 regulates microRNAs and cell growth in the Drosophila ovarian stem cell lineage. Nature 454, 241–245 (2008).
Schwamborn, J. C., Berezikov, E. & Knoblich, J. A. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 136, 913–925 (2009).
Weinmann, L. et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell 136, 496–507 (2009).
Pare, J. M. et al. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol. Biol. Cell 20, 3273–3284 (2009).
Iki, T. et al. In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol. Cell 3 Jun 2010 (doi:10.1016/j.molcel.2010.05.014).
Iwasaki, S. et al. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol. Cell 3 Jun 2010 (doi:10.1016/j.molcel.2010.05.015).
Kedde, M. et al. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131, 1273–1286 (2007).
Mishima, Y. et al. Differential regulation of germline mRNAs in soma and germ cells by zebrafish miR-430. Curr. Biol. 16, 2135–2142 (2006).
Nolde, M. J., Saka, N., Reinert, K. L. & Slack, F. J. The Caenorhabditis elegans pumilio homolog, puf-9, is required for the 3′UTR-mediated repression of the let-7 microRNA target gene, hbl-1. Dev. Biol. 305, 551–563 (2007).
Galgano, A. et al. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One 3, e3164 (2008).
Kim, H. H. et al. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23, 1743–1748 (2009).
Eiring, A. M. et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 140, 652–665 (2010).
Vasudevan, S. & Steitz, J. A. A U-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 128, 1105–1118 (2007).
Vasudevan, S., Tong, Y. & Steitz, J. A. Switching from repression to activation: microRNAs can up-regulate translation. Science 318, 1931–1934 (2007).
Orom, U. A., Nielsen, F. C. & Lund, A. H. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 30, 460–471 (2008).
Henke, J. I. et al. microRNA-122 stimulates translation of hepatitis C virus RNA. Embo J. 27, 3300–3310 (2008).
Lee, Y. S. et al. Silencing by small RNAs is linked to endosomal trafficking. Nature Cell Biol. 11, 1150–1156 (2009).
Cikaluk, D. E. et al. GERp95, a membrane-associated protein that belongs to a family of proteins involved in stem cell differentiation. Mol. Biol. Cell 10, 3357–3372 (1999).
Tahbaz, N. et al. Characterization of the interactions between mammalian PAZ PIWI domain proteins and Dicer. EMBO Rep. 5, 189–194 (2004).
Carlsbecker, A. et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465, 316–321 (2010).
Rechavi, O. et al. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 23, 1971–1979 (2009).
Meister, G. et al. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15, 185–197 (2004).
Robb, G. B., Brown, K. M., Khurana, J. & Rana, T. M. Specific and potent RNAi in the nucleus of human cells. Nature Struct. Mol. Biol. 12, 133–137 (2005).
Rudel, S., Flatley, A., Weinmann, L., Kremmer, E. & Meister, G. A multifunctional human Argonaute2-specific monoclonal antibody. Rna 14, 1244–1253 (2008).
Castanotto, D., Lingeman, R., Riggs, A. D. & Rossi, J. J. CRM1 mediates nuclear-cytoplasmic shuttling of mature microRNAs. Proc. Natl Acad. Sci. USA 106, 21655–21659 (2009).
Till, S. et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nature Struct. Mol. Biol. 14, 897–903 (2007).
Khraiwesh, B. et al. Transcriptional control of gene expression by microRNAs. Cell 140, 111–122 (2010).
Bao, N., Lye, K. W. & Barton, M. K. MicroRNA binding sites in Arabidopsis class III HD-ZIP mRNAs are required for methylation of the template chromosome. Dev. Cell 7, 653–662 (2004).
Wu, L. et al. DNA methylation mediated by a microRNA pathway. Mol. Cell 38, 465–475 (2010).
Kim, D. H., Saetrom, P., Snove, O. & Rossi, J. J. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc. Natl Acad. Sci. USA 105, 16230–16235 (2008).
Ashraf, S. I., McLoon, A. L., Sclarsic, S. M. & Kunes, S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124, 191–205 (2006).
Banerjee, S., Neveu, P. & Kosik, K. S. A coordinated local translational control point at the synapse involving relief from silencing and MOV10 degradation. Neuron 64, 871–884 (2009).
Schratt, G. M. et al. A brain-specific microRNA regulates dendritic spine development. Nature 439, 283–289 (2006).
Siegel, G. et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nature Cell Biol. 11, 705–716 (2009). References 121–124 demonstrated that neuronal activation triggers the local relief of miRNA-meditated repression of translation at dendritic spines, contributing to synaptic plasticity and long-term potentiation.
Barbee, S. A. et al. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron 52, 997–1009 (2006).
Cougot, N. et al. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J. Neurosci. 28, 13793–13804 (2008).
Zeitelhofer, M. et al. Dynamic interaction between P-bodies and transport ribonucleoprotein particles in dendrites of mature hippocampal neurons. J. Neurosci. 28, 7555–7562 (2008).
Lugli, G., Torvik, V. I., Larson, J. & Smalheiser, N. R. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J. Neurochem. 106, 650–661 (2008).
Li, X. & Jin, P. Macro role(s) of microRNAs in fragile X syndrome? Neuromolecular Med. 11, 200–207 (2009).
Edbauer, D. et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65, 373–84.
Jin, P. et al. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nature Neurosci. 7, 113–117 (2004).
van Rooij, E. et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316, 575–579 (2007).
Gatfield, D. et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 23, 1313–1326 (2009).
Krol, J. et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141, 618–631 (2010). Reports that many miRNAs in neurons have a very rapid turnover, which is regulated by neuronal activity.
Hwang, H. W., Wentzel, E. A. & Mendell, J. T. A hexanucleotide element directs microRNA nuclear import. Science 315, 97–100 (2007).
Buck, A. H. et al. Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection. RNA 16, 307–315 (2010).
Sethi, P. & Lukiw, W. J. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer's disease temporal lobe neocortex. Neurosci. Lett. 459, 100–104 (2009).
Rajasethupathy, P. et al. Characterization of small RNAs in aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63, 803–817 (2009).
Kocerha, J. et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc. Natl Acad. Sci. USA 106, 3507–3512 (2009).
Wibrand, K. et al. Differential regulation of mature and precursor microRNA expression by NMDA and metabotropic glutamate receptor activation during LTP in the adult dentate gyrus in vivo. Eur. J. Neurosci. 31, 636–645 (2010).
Flavell, S. W. & Greenberg, M. E. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu. Rev. Neurosci. 31, 563–590 (2008).
Katoh, T. et al. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev. 23, 433–438 (2009).
Lu, S., Sun, Y. H. & Chiang, V. L. Adenylation of plant miRNAs. Nucleic Acids Res. 37, 1878–1885 (2009).
Li, J., Yang, Z., Yu, B., Liu, J. & Chen, X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 15, 1501–1507 (2005).
Ramachandran, V. & Chen, X. Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 321, 1490–1492 (2008).
Chatterjee, S. & Grosshans, H. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature 461, 546–549 (2009). References 145 and 146 identified the first exoribonucleases known to be involved in degradation of mature miRNAs.
Chi, S. W., Zang, J. B., Mele, A. & Darnell, R. B. Argonaute HITS-CLIP decodes microRNA–mRNA interaction maps. Nature 460, 479–486 (2009).
Mayr, C. & Bartel, D. P. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138, 673–684 (2009).
Sandberg, R., Neilson, J. R., Sarma, A., Sharp, P. A. & Burge, C. B. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320, 1643–1647 (2008).
Edbauer, D. et al. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65, 373–384 (2010).
Chekulaeva, M., Filipowicz, W. & Parker, R. Multiple independent domains of dGW182 function in miRNA-mediated repression in Drosophila. RNA 15, 794–803 (2009).
Ozsolak, F. et al. Chromatin structure analyses identify miRNA promoters. Genes Dev. 22, 3172–3183 (2008).
Corcoran, D. L. et al. Features of mammalian microRNA promoters emerge from polymerase II chromatin immunoprecipitation data. PLoS One 4, e5279 (2009).
O'Donnell, K. A., Wentzel, E. A., Zeller, K. I., Dang, C. V. & Mendell, J. T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435, 839–843 (2005).
Ma, L. et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nature Cell Biol. 12, 247–256 (2010).
Chang, T. C. et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nature Genet. 40, 43–50 (2008).
He, L. et al. A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134 (2007).
Conaco, C., Otto, S., Han, J. J. & Mandel, G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl Acad. Sci. USA 103, 2422–2427 (2006).
Han, L., Witmer, P. D., Casey, E., Valle, D. & Sukumar, S. DNA methylation regulates microRNA expression. Cancer Biol. Ther. 6, 1284–1288 (2007).
Eulalio, A., Behm-Ansmant, I. & Izaurralde, E. P bodies: at the crossroads of post-transcriptional pathways. Nature Rev. Mol. Cell Biol. 8, 9–22 (2007).
Franks, T. M. & Lykke-Andersen, J. The control of mRNA decapping and P-body formation. Mol. Cell 32, 605–615 (2008).
Parker, R. & Sheth, U. P bodies and the control of mRNA translation and degradation. Mol. Cell 25, 635–646 (2007).
Chu, C. Y. & Rana, T. M. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4, e210 (2006).
Eulalio, A., Behm-Ansmant, I., Schweizer, D. & Izaurralde, E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27, 3970–3981 (2007).
Pauley, K. M. et al. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 7, 904–910 (2006).
Huang, J. et al. Derepression of microRNA-mediated protein translation inhibition by apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) and its family members. J. Biol. Chem. 282, 33632–33640 (2007).
Liu, J., Valencia-Sanchez, M. A., Hannon, G. J. & Parker, R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nature Cell Biol. 7, 719–723 (2005).
Pillai, R. S. et al. Inhibition of translational initiation by Let-7 microRNA in human cells. Science 309, 1573–1576 (2005).
Nathans, R. et al. Cellular microRNA and P bodies modulate host–HIV-1 interactions. Mol. Cell 34, 696–709 (2009).
Ma, J. et al. MicroRNA activity is suppressed in mouse oocytes. Curr. Biol. 20, 265–270 (2010).
Suh, N. et al. MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr. Biol. 20, 271–277 (2010).
Flemr, M., Ma, J., Schultz, R. M. & Svoboda, P. P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol. Reprod. 82, 1008–1017 (2010).
Swetloff, A. et al. Dcp1-bodies in mouse oocytes. Mol. Biol. Cell 20, 4951–4961 (2009).
Kedersha, N. et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884 (2005).
Sakamoto, S. et al. The NF90–NF45 complex functions as a negative regulator in the microRNA processing pathway. Mol. Cell. Biol. 29, 3754–3769 (2009).
Watanabe, M. et al. A subfamily of RNA-binding DEAD-box proteins acts as an estrogen receptor alpha coactivator through the N-terminal activation domain (AF-1) with an RNA coactivator, SRA. EMBO J. 20, 1341–1352 (2001).
Yamagata, K. et al. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol. Cell 36, 340–347 (2009).
Acknowledgements
We thank the members of the W.F. group for valuable discussion and comments on the manuscript. J.K. is a recipient of an EMBO Fellowship. I.L. is supported by Deutsche Forschungsgemeinschaft (DFG). The Friedrich Miescher Institute is supported by the Novartis Research Foundation. Research by W.F. is also supported by the EC FP6 Program “Sirocco”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Regulators of miRNA processing and function (PDF 320 kb)
Related links
Glossary
- Deadenylation
-
The removal of the poly(A) tail from the mRNA 3′ end. Deadenylation is the first step in mRNA decay, and is generally followed by removal of the m7G cap (the 7-methylguanosine-triphosphate structure at the 5′ end of mRNAs, which promotes their translation and protects them from degradation) and exonucleolytic 5′ to 3′ degradation of mRNA. Deadenylation is mainly mediated by the CAF1–CCR4 deadenylase complex.
- Seed sequence
-
Nucleotide positions 2–8 from the 5′ end of the microRNA, which generally perfectly base-pair with target mRNA, and are important for defining the target repertoire of a microRNA.
- Allosteric regulation
-
A mechanism by which an event at one region in a protein causes an effect at another site.
- RNP
-
(Ribonucleoprotein). A complex of RNA and proteins. In the case of mRNPs the complex assembles on mRNA; in the case of miRNPs (also known as microRNA-induced silencing complexes), this involves microRNAs instead.
- 3′-UTR
-
The 3′-UTR controls many aspects of mRNA metabolism, such as transport, localization, efficiency of translation and stability. 3′-UTRs can extend over several kilobases and generally contain binding sites for various regulatory proteins and microRNAs allowing dynamic and combinatorial regulation.
- CLIP
-
(Crosslinking immunoprecipitation). CLIP technology facilitates the identification and sequencing of short RNA regions associating with RNA-binding proteins or with microRNA-induced silencing complexes in intact cells.
Rights and permissions
About this article
Cite this article
Krol, J., Loedige, I. & Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11, 597–610 (2010). https://doi.org/10.1038/nrg2843
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2843
This article is cited by
-
The role of ADAR1 through and beyond its editing activity in cancer
Cell Communication and Signaling (2024)
-
HIF-2α/LINC02609/APOL1-mediated lipid storage promotes endoplasmic reticulum homeostasis and regulates tumor progression in clear-cell renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2024)
-
Exosomal microRNAs in Parkinson’s disease: insights into biomarker potential and disease pathology
Neurological Sciences (2024)
-
Electroacupuncture Promotes Nerve Regeneration and Functional Recovery Through Regulating lncRNA GAS5 Targeting miR-21 After Sciatic Nerve Injury
Molecular Neurobiology (2024)
-
Identification of Differentially Expressed mRNAs and miRNAs and Related Regulatory Networks in Cumulus Oophorus Complexes Associated with Fertilization
Reproductive Sciences (2024)