Abstract
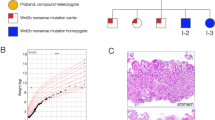
Hirschsprung disease (HSCR), or congenital aganglionic megacolon, is the most common cause of congenital bowel obstruction with an incidence of 1 in 5000 live births1. HSCR may be inherited as a single gene disorder with reduced penetrance or as a multigenic trait2. HSCR mutations have been identified in the RET receptor tyrosine kinase3–6, endothelin-B receptor (EDNRB) and its physiological ligand, endothelin 3 (EDN3). Although RET's ligand has remained elusive, it is expected to be an extracellular neurotrophic molecule expressed in the developing gut and kidney mesenchyme, based on the phenotypes of intestinal aganglionosis and renal agenesis observed in homozygous RET knockout (Ret−/−) mice7,8. The glial cell line-derived neurotrophic factor (GDNF) is such a molecule. Recently, mice carrying two null alleles for Gdnf were shown to exhibit phenotypes remarkably similar to Ret−/− animals9–11. We screened 106 unrelated HSCR patients for mutations in GDNF by direct sequencing. We identified one familial mutation in a HSCR patient with a known de novo RET mutation and malrotation of the gut. No haplotype sharing was evident in any of 36 HSCR kindreds typed for microsatellite markers surrounding GDNF on human chromosome 5p. Our data suggest that GDNF is a minor contributor to human HSCR susceptibility and that loss of its function in enteric neurogenesis may be compensated for by other neurotrophic factors or via other pathways. However, it may be that in rare instances, RET and GDNF mutations act in concert to produce an enteric phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Holschneider, A.M. (ed.) Hirschsprung's disease. (Thieme-Stratton, New York, 1982).
Badner, J.A., Sieber, W.K., Garver, K.L. & Chakravarti, A.A. . A genetic study of Hirschsprung disease. Am. J. Hum. Genet. 46, 565–580 (1990).
Angrist, M. et al. Mutation analysis of the RET receptor tyrosine kinase in Hirschsprung disease. Hum. Mol. Genet. 4, 821–830 (1995).
Attié, T. et al. Diversity of RET proto-oncogene mutations in familial and sporadic Hirschsprung disease. Hum. Mol. Genet. 4, 1381–1386 (1995).
Yin, L. et al. Heterogeneity and low detection rate of RET mutations in Hirschsprung disease. Eur. J. Hum. Genet. 2, 272–280 (1994).
Mulligan, L.M. et al. Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum. Mol. Genet. 3, 2163–2167 (1994).
Dressler, G.R. The pros and cons of c-ret. Curr. Biol. 4, 354–356 (1994).
Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994).
Moore, M.W. et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382, 76–79 (1996).
Pichel, J.G. et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382, 73–76 (1996).
Sánchez, M.P. et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382, 70–73 (1996).
Bodian, M. & Carter, C.O. A family study of Hirschsprung's disease. Ann. Hum. Genet. 28, 261–277 (1963).
Angrist, M. et al. A gene for Hirschsprung disease (megacoton) in the pericentromeric region of chromosome 10. Nature Genet. 4, 351–356 (1993).
Lyonnet, S. et al. A gene for Hirschsprung disease maps to the proximal long arm of chromosome 10. Nature Genet. 4, 346–350 (1993).
Puffenberger, E.G. et al. Identity-by-descent and association mapping of a recessive gene for Hirschsprung disease on human chromosome 13q22. Hum. Mol. Genet. 3, 1217–1225 (1994).
Edery, P. et al. Mutations of the RET proto-oncogene in Hirschsprung's disease. Nature 367, 378–380 (1994).
Romeo, G. et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature 367, 377–378 (1994).
Puffenberger, E.G. et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung disease. Cell 79, 1257–1266 (1994).
Edery, P. et al. Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease (Shah-Waardenburg syndrome) Nature Genet. 12, 442–444 (1996).
Hofstra, R.M.W. et al. A homozygous mutation in the endothelin-3 gene associated with a combined Waardenburg type 2 and Hirschsprung phenotype (Shah-Waardenburg syndrome). Nature Genet. 12, 445–447 (1996).
Takahashi, M. et al. Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 3, 571–578 (1988).
Pasini, B., Ceccherini, I. & Romeo, G. RET mutations in human disease. Trends Genet. 12, 138–144 (1996).
Durbec, P.L., Larsson-Blomberg, L.B., Schuchardt, A., Costantini, F. & Pachnis, V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122, 349–358 (1996).
Lin, L.-F.H. et al. GDNF: a glial cell line–derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 (1993).
Lapchak, P.A. Therapeutic potentials for glial cell line-derived neurotrophic factor (GDNF) based upon pharmacological activities in the CNS. Rev. Neurosci. (in the press).
Buj-Bello, A. et al. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron 15, 821–828 (1995).
Henderson, C.E. et al. GDNF: a potent survival factor for motoneurons in peripheral nerve and muscle. Science 266, 1062–1064 (1994).
Oppenheim, R.W. et al. Developing motor neurons rescued from programmed axotomy-induced cell death by GDNF. Nature 373, 344–346 (1995).
Yan, Q., Matheson, C. & Lopez, O.T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature 373, 341–344 (1995).
Zurn, A.D. et al. Glial cell line-derived neurotrophic factor (GDNF), a new neurotrophic factor for motoneurones. Neuroreport 6, 113–118 (1994).
Hellmich, H.L. et al. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech. Dev. 54, 95–105 (1996).
Kingsley, D.M., TGF-beta superfamily—new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 8, 133–146 (1994).
Bermingham, N. et al. Human glial cell line-derived neurotrophic factor (GDNF) maps to chromosome 5. Hum. Genet. 96, 671–673 (1995).
Schindelhauer, D. et al. The gene coding for glial cell line derived neurotrophic factor (GDNF) maps to chromosome 5p12–p13 Genomics 28, 605–607 (1995).
Durbec, P. et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature 381, 789–793 (1996).
Jing, S. et al. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 85, 1113–1124 (1996).
Treanor, J.J.S. et al. Characterization of a multicomponent receptor for GDNF. Nature 382, 80–83 (1996).
Trupp, M. et al. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature 381, 785–789 (1996).
Vyse, T.J. & Todd, J.A. Genetic analysis of autoimmune disease. Cell 85, 311–318 (1996).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380, 152–154 (1996).
Barbacid, M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann. N. Y. Acad. Sci. 766, 442–458 (1995).
Muenke, M. & Schell, U. Fibroblast-growth-factor receptor mutations in human skeletal disorders. Trends Genet. 11, 308–313 (1995).
Ezoe, K. et al. Novel mutations and deletions of the KIT (steel factor receptor) gene in human piebaldism. Am. J. Hum. Genet. 56, 58–66 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angrist, M., Bolk, S., Halushka, M. et al. Germline mutations in glial cell line-derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient. Nat Genet 14, 341–344 (1996). https://doi.org/10.1038/ng1196-341
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1196-341
This article is cited by
-
Genetic background-dependent abnormalities of the enteric nervous system and intestinal function in Kif26a-deficient mice
Scientific Reports (2021)
-
Defining the transcriptomic landscape of the developing enteric nervous system and its cellular environment
BMC Genomics (2017)
-
Hereditary syndromes predisposing to endocrine tumors and their skin manifestations
Reviews in Endocrine and Metabolic Disorders (2016)
-
RET gene is a major risk factor for Hirschsprung’s disease: a meta-analysis
Pediatric Surgery International (2015)
-
Knockout mouse models of Hirschsprung’s disease
Pediatric Surgery International (2015)