Abstract
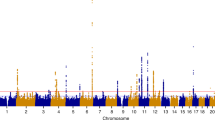
Although the pathogenesis of endometriosis is not well understood, genetic factors have been considered to have critical roles in its etiology. Through a genome-wide association study and a replication study using a total of 1,907 Japanese individuals with endometriosis (cases) and 5,292 controls, we identified a significant association of endometriosis with rs10965235 (P = 5.57 × 10−12, odds ratio = 1.44), which is located in CDKN2BAS on chromosome 9p21, encoding the cyclin-dependent kinase inhibitor 2B antisense RNA. By fine mapping, the SNP showing the strongest association was located in intron 16 of CDKN2BAS and was implicated in regulating the expression of p15, p16 and p14. A SNP, rs16826658, in the LD block including WNT4 on chromosome 1p36, which is considered to play an important role in the development of the female genital tract, revealed a possible association with endometriosis (P = 1.66 × 10−6, odds ratio = 1.20). Our findings suggest that these regions are new susceptibility loci for endometriosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mori, H., Taketani, Y., Uemura, T., Miyake, A. & Tango, T. Rates of endometriosis recurrence and pregnancy 1 year after treatment with intranasal buserelin acetate (Suprecur) (a prospective study). J. Obstet. Gynaecol. Res. 25, 153–164 (1999).
Stern, R.C. et al. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int. J. Gynecol. Pathol. 20, 133–139 (2001).
Bulun, S.E. Endometriosis. N. Engl. J. Med. 360, 268–279 (2009).
Giudice, L.C. & Kao, L.C. Endometriosis. Lancet 364, 1789–1799 (2004).
Sampson, J.A. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 3, 93–110.43 (1927).
Matalliotakis, I.M. et al. Familial aggregation of endometriosis in the Yale Series. Arch. Gynecol. Obstet. 278, 507–511 (2008).
Seracchioli, R. et al. Post-operative use of oral contraceptive pills for prevention of anatomical relapse or symptom-recurrence after conservative surgery for endometriosis. Hum. Reprod. 24, 2729–2735 (2009).
Juo, S.H. et al. CYP17, CYP1A1 and COMT polymorphisms and the risk of adenomyosis and endometriosis in Taiwanese women. Hum. Reprod. 21, 1498–1502 (2006).
Vietri, M.T. et al. CYP17 and CYP19 gene polymorphisms in women affected with endometriosis. Fertil. Steril. 92, 1532–1535 (2009).
Sato, H. et al. Intron 1 and exon 1 alpha estrogen receptor gene polymorphisms in women with endometriosis. Fertil. Steril. 90, 2086–2090 (2008).
Shan, K. et al. The function of the SNP in the MMP1 and MMP3 promoter in susceptibility to endometriosis in China. Mol. Hum. Reprod. 11, 423–427 (2005).
Han, Y.J. et al. Haplotype analysis of the matrix metalloproteinase-9 gene associated with advanced-stage endometriosis. Fertil. Steril. 91, 2324–2330 (2009).
Jarinova, O. et al. Functional analysis of the chromosome 9p21.3 coronary artery disease risk locus. Arterioscler. Thromb. Vasc. Biol. 29, 1671–1677 (2009).
Liu, Y. et al. INK4/ARF transcript expression is associated with chromosome 9p21 variants linked to atherosclerosis. PLoS One 4, e5027 (2009).
Pasmant, E. et al. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 67, 3963–3969 (2007).
Kool, J. et al. Insertional mutagenesis in mice deficient for p15Ink4b, p16Ink4a, p21Cip1, and p27Kip1 reveals cancer gene interactions and correlations with tumor phenotypes. Cancer Res. 70, 520–531 (2010).
Okuda, T. et al. Molecular cloning, expression pattern, and chromosomal localization of human CDKN2D/INK4d, an inhibitor of cyclin D-dependent kinases. Genomics 29, 623–630 (1995).
Nakashima, R. et al. Alteration of p16 and p15 genes in human uterine tumours. Br. J. Cancer 80, 458–467 (1999).
Yanokura, M. et al. Hypermethylation in the p16 promoter region in the carcinogenesis of endometrial cancer in Japanese patients. Anticancer Res. 26, 851–856 (2006).
Ignatov, A. et al. P16 alterations increase the metastatic potential of endometrial carcinoma. Gynecol. Oncol. 111, 365–371 (2008).
Martini, M. et al. Possible involvement of hMLH1, p16(INK4a) and PTEN in the malignant transformation of endometriosis. Int. J. Cancer 102, 398–406 (2002).
Guida, M. et al. Aberrant DNA hypermethylation of hMLH-1 and CDKN2A/p16 genes in benign, premalignant and malignant endometrial lesions. Eur. J. Gynaecol. Oncol. 30, 267–270 (2009).
Goumenou, A.G., Arvanitis, D.A., Matalliotakis, I.M., Koumantakis, E.E. & Spandidos, D.A. Loss of heterozygosity in adenomyosis on hMSH2, hMLH1, p16Ink4 and GALT loci. Int. J. Mol. Med. 6, 667–671 (2000).
Samani, N.J. et al. Genome-wide association analysis of coronary artery disease. N. Engl. J. Med. 357, 443–453 (2007).
Shen, G.Q. et al. Association between four SNPs on chromosome 9p21 and myocardial infarction is replicated in an Italian population. J. Hum. Genet. 53, 144–150 (2008).
Scott, L.J. et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316, 1341–1345 (2007).
Silander, K. et al. Worldwide patterns of haplotype diversity at 9p21.3, a locus associated with type 2 diabetes and coronary heart disease. Genome Med. 1, 51 (2009).
Horikawa, Y. et al. Replication of genome-wide association studies of type 2 diabetes susceptibility in Japan. J. Clin. Endocrinol. Metab. 93, 3136–3141 (2008).
Helgadottir, A. et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat. Genet. 40, 217–224 (2008).
Yasuno, K. et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat. Genet. 42, 420–425 (2010).
Bishop, D.T. et al. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet. 41, 920–925 (2009).
Stacey, S.N. et al. New common variants affecting susceptibility to basal cell carcinoma. Nat. Genet. 41, 909–914 (2009).
Falchi, M. et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat. Genet. 41, 915–919 (2009).
Shete, S. et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat. Genet. 41, 899–904 (2009).
Cunnington, M.S., Santibanez Koref, M., Mayosi, B.M., Burn, J. & Keavney, B. Chromosome 9p21 SNPs associated with multiple disease phenotypes correlate with ANRIL expression. PLoS Genet. 6, e1000899 (2010).
Shen, G.Q. et al. Four SNPs on chromosome 9p21 in a South Korean population implicate a genetic locus that confers high cross-race risk for development of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 28, 360–365 (2008).
Omori, S. et al. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes 57, 791–795 (2008).
Gaetje, R. et al. Endometriosis may be generated by mimicking the ontogenetic development of the female genital tract. Fertil. Steril. 87, 651–656 (2007).
Vinatier, D., Orazi, G., Cosson, M. & Dufour, P. Theories of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 96, 21–34 (2001).
Vainio, S., Heikkila, M., Kispert, A., Chin, N. & McMahon, A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405–409 (1999).
Parr, B.A. & McMahon, A.P. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395, 707–710 (1998).
Stark, K., Vainio, S., Vassileva, G. & McMahon, A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).
Babu, K.A. et al. N-acetyl transferase 2 polymorphism and advanced stages of endometriosis in South Indian women. Reprod. Biomed. Online 9, 533–540 (2004).
Nakamura, Y. The BioBank Japan Project. Clin. Adv. Hematol. Oncol. 5, 696–697 (2007).
Ohnishi, Y. et al. A high-throughput SNP typing system for genome-wide association studies. J. Hum. Genet. 46, 471–477 (2001).
de Bakker, P.I. et al. Efficiency and power in genetic association studies. Nat. Genet. 37, 1217–1223 (2005).
Barrett, J.C., Fry, B., Maller, J. & Daly, M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Acknowledgements
We thank the members of the Rotary Club of Osaka-Midosuji District 2660 Rotary International in Japan for supporting our study. We also thank the technical staff of the Laboratory for Genotyping Development in RIKEN, Laboratory of Molecular Medicine, Human Genome Center, Institute of Medical Science, The University of Tokyo and Department of Obstetrics and Gynecology, Keio University, School of Medicine. This work was conducted as a part of the BioBank Japan Project that was supported by the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government.
Author information
Authors and Affiliations
Contributions
Y.N. conceived the study. Y.N., H.Z., S.U., K.H. and M.K. designed the study. S.U., T.A. and M.K. performed genotyping. S.U., A.T., N.K. and M.K. performed the data analyses. Y.N., H.Z., and M.K. managed DNA samples belonging to BioBank Japan. A.H. and D.A. managed DNA samples belonging to Keio University. S.U. summarized the results. S.U., H.Z. and Y.N. wrote the manuscript. Y.N. obtained funding for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Tables 1–3 (PDF 666 kb)
Rights and permissions
About this article
Cite this article
Uno, S., Zembutsu, H., Hirasawa, A. et al. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet 42, 707–710 (2010). https://doi.org/10.1038/ng.612
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.612
This article is cited by
-
Integration of genome-wide association study and expression quantitative trait locus mapping for identification of endometriosis-associated genes
Scientific Reports (2021)
-
GWAS of five gynecologic diseases and cross-trait analysis in Japanese
European Journal of Human Genetics (2020)
-
Endometriosis, endocrine disrupters, and epigenetics: an investigation into the complex interplay in women with polybrominated biphenyl exposure and endometriosis
Journal of Assisted Reproduction and Genetics (2020)
-
Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis
Nature Communications (2019)
-
Japanese GWAS identifies variants for bust-size, dysmenorrhea, and menstrual fever that are eQTLs for relevant protein-coding or long non-coding RNAs
Scientific Reports (2018)